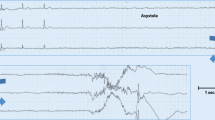
Abstract
Purpose: We tested whether the adenosine A1 receptor agonist, R-PIA, suppressed torsade de pointes (TdP) induced by the delayed rectifier potassium channel blocker clofilium. Furthermore, we studied the underlying mechanism: β-adrenergic antagonism or ATP-sensitive K+ channel (IK-ATP) opening. Methods: In anesthetized rabbits, TdP was induced by simultaneous infusion of clofilium and the α1-adrenoceptor agonist methoxamine. Four groups were studied: (1) saline infusion after TdP induction; (2) R-PIA (1.3 mg/kg) infusion; (3) R-PIA infusion after propranolol (2 µmol/kg) pretreatment; (4) R-PIA infusion after glibenclamide (10 µmol/kg) pretreatment. Results: TdP suppression rate was 0% in group 1, 78% in group 2 (p < 0.01 vs group 1), 67% in group 3 (p < 0.05 vs group 1, p = NS vs group 2), 33% in group 4 (p = NS vs group 1, p = 0.08 vs group 2). TdP induction coincided with increased QT/QTc duration and QT dispersion. TdP suppression coincided with reduced QT dispersion, but further QT/QTc lengthening. Conclusions: R-PIA suppressed TdP, not by β-adrenergic antagonism, but mostly by IK-ATP opening. QT dispersion correlated better with TdP induction/suppression than QT/QTc duration.
Similar content being viewed by others
References
Tan HL, Hou CJY, Lauer MR, Sung RJ. Electrophysiologic mechanisms of the long QT interval syndromes and torsade de pointes. Ann Intern Med 1995;122:701–714.
Brachmann J, Scherlag BJ, Rosenshtraukh LV, Lazzara R. Bradycardia-dependent triggered activity: relevance to drug-induced multiform ventricular tachycardia. Circulation 1983;68:846–856.
El-Sherif N, Caref EB, Yin H, Restivo M. The electrophysiological mechanism of ventricular arrhythmias in the long QT syndrome: tridimensional mapping of activation and recovery patterns. Circ Res 1996;79:474–492.
Carlsson L, Abrahamsson C, Drews L, Duker G. Antiarrhythmic effects of potassium channel openers in rhythm abnormalities related to delayed repolarization. Circulation 1992;85:1491–1500.
Chinushi M, Aizawa Y, Furushima H, Inuzuka H, Ojima K, Shibata A. Nicorandil suppresses a hump on the monophasic action potential and torsade de pointes in a patient with idiopathic long QT syndrome. Jpn Heart J 1995;36:477–481.
Fish FA, Prakash C, Roden DM. Suppression of repolarization-related arrhythmias in vitro and in vivo by low-dose potassium channel activators. Circulation 1990;82:1362–1369.
Sato T, Hata Y, Yamamoto M, Morita H, Mizuo K, Yamanari H. Early afterdepolarization abolished by potassium channel opener in a patient with idiopathic long QT syndrome. J Cardiovasc Electrophysiol 1995;6:279–282.
Takahashi N, Ito M, Saikawa T, Arita M. Nicorandil suppresses early afterdepolarisation and ventricular arrhythmias induced by caesium chloride in rabbits in vivo. Cardiovasc Res 1991;25:445–452.
Takahashi N, Ito M, Ishida S, Fujino T, Saikawa T, Arita M. Effects of vagal stimulation on cesium-induced early afterdepolarizations and ventricular arrhythmias in rabbits. Circulation 1992;86:1987–1992.
Carlsson L, Drews L, Duker G, Schiller-Linhardt G. Attenuation of proarrhythmias related to delayed repolarization by low-dose lidocaine in the anaesthetized rabbit. J Pharmacol Exp Ther 1993;267:1076–1080.
Nattel S, Quantz MA. Pharmacological response of quinidine induced early afterdepolarizations in canine cardiac Purkinje fibres: Insights into underlying ionic mechanisms. Cardiovasc Res 1988;22:808–817.
Roden DM, Hoffmann BF. Action potential prolongation and induction of abnormal automaticity by low quinidine concentrations in canine Purkinje fibers. Relationship to potassium and cycle length. Circ Res 1985;56:857–867.
January CT, Riddle JM. Early afterdepolarizations: mechanism of induction and block. A role for L-type Ca2+ current. Circ Res 1994;64:977–990.
Song Y, Thedford S, Lerman BB, Belardinelli L. Adenosine-sensitive afterdepolarizations and triggered activity in guinea pig ventricular myocytes. Circ Res 1992;70:743–753.
Lerman BB, Belardinelli L. Cardiac electrophysiology of adenosine. Basic and clinical concepts. Circulation 1991;83:1499–1509.
Kim E, Han J, Ho W, Earm YE. Modulation of ATP-sensitive K+ channels in rabbit ventricular myocytes by adenosine A1 receptor activation. Am J Physiol 1997;272:H325–H333.
Liu Y, Gao WD, O'Rourke B, Marban E. Synergistic modulation of ATP-sensitive K + currents by protein kinase C and adenosine. Implications for ischemic preconditioning. Circ Res 1996;78:443–454.
Kirsch GE, Codina J, Birnbaumer L, Brown AM. Coupling of ATP-sensitive K + channels to A1 receptors by G proteins in rat ventricular myocytes. Am J Physiol 1990;259:H820–H826.
Ito H, Vereecke J, Carmeliet E. Mode of regulation by G protein of the ATP-sensitive K + channel in guinea pig ventricular cell membrane. J Physiol (London) 1994;478:101–108.
Daly JW, Jacobson KA, Ukena D. Adenosine receptors: Development of selective agonists and antagonists. Progr Clin Biol Res 1987;230:41–63.
Carlsson L, Drews L, Duker G. Rhythm anomalies related to delayed repolarization in vivo: Influence of sarcolemmal Ca + + entry and intracellular Ca + + overload. J Pharmacol Exp Ther 1996;279:231–239.
Toombs CF, McGee DS, Johnston WE, Vinten-Johansen W. Protection from ischaemic-reperfusion injury with adenosine pretreatment is reversed by inhibition of ATP sensitive potassium channels. Cardiovasc Res 1993;27:623–629.
Compton SJ, Lux RL, Ramsey MR, et al. Genetically defined therapy of inherited long-QT syndrome: Correction of abnormal repolarization by potassium. Circulation 1996;94:1018–1022.
Priori SG, Napolitano C, Diehl L, Schwartz PJ. Dispersion of the QT interval: A marker of therapeutic efficacy in the idiopathic long QT syndrome. Circulation 1994;89:1681–1689.
Belardinelli L, Isenberg G. Isolated atrial myocytes: adenosine and acetylcholine increase potassium conductance. Am J Physiol 1983;244:H734–H737.
Kurachi Y, Nakajima T, Sugimoto T. On the mechanism of activation of muscarinic K + channels by adenosine in isolated atrial cells: involvement of GTP-binding proteins. Pfluegers Arch 1986;407:264–274.
Li GR, Feng J, Schrier A, Nattel S. Contribution of ATP-sensitive potassium channels to the electrophysiological effects of adenosine in guinea-pig atrial cells. J Physiol (London) 1995;484:629–642.
Isenberg G, Belardinelli L. Ionic basis for the antagonism between adenosine and isoproterenol on isolated mammalian ventricular myocytes. Circ Res 1984;55:309–325.
Gupta RC, Neumann J, Durant P, Watanabe AM. A1-adenosine receptor-mediated inhibition of isoproterenol-stimulated protein phosphorylation in ventricular myocytes. Evidence against a cAMP-dependent effect. Circ Res 1993;72:65–74.
Nayebpour M, Nattel S. Pharmacologic response of cesium-induced ventricular tachyarrhythmias in anesthetized dogs. J Cardiovasc Pharmacol 1990;15:552–561.
Belardinelli L, Shryock JC, Song Y, Wang D, Srinivas M. Ionic basis of the electrophysiological actions of adenosine on cardiomyocytes. FASEB J 1995;9:359–365.
Koumi S, Wasserstrom JA. Acetylcholine-sensitive muscarinic K + channels in mammalian ventricular myocytes. Am J Physiol 1994;266:H1812–H1821.
Yao Z, Gross GJ. A comparison of adenosine-induced cardioprotection and ischemic preconditioning in dogs: efficacy, time course, and role of KATP channels. Circulation 1994;89:1229–1236.
Song Y, Belardinelli L. Evidence against the existence of adenosine-induced activation of ATP-sensitive K + channels in guinea pig cardiac myocytes (Abstract). J Am Coll Cardiol 1994;23(Suppl):827–3??
Song Y, Belardinelli L. Electrophysiological and functional effects of adenosine on ventricular myocytes of various mammalian species. Am J Physiol 1996;271:C1233–C1243.
Wesley RC, Belardinelli L. Role of adenosine on ventricular overdrive suppression in isolated guinea pig hearts and Purkinje fibers. Circ Res 1985;57:517–531.
Boachie-Ansah G, Kane KA, Parratt JR. Electrophysiological effects of adenosine and adenosine triphosphate on sheep Purkinje fibres under normal and simulated ischemic conditions. Br J Pharmacol 1989;97:240–246.
Rardon DP, Bailey JC. Adenosine attenuation of the electrophysiological effects of isoproterenol on canine cardiac Purkinje fibers. J Pharmacol Exp Ther 1984;228:792–798.
Day CP, McComb JM, Campbell RWF. QT dispersion: An indicator of arrhythmia risk in patients with long QT intervals. Br Heart J 1990;63:342–344.
Hii JT, Wyse DG, Gillis AM, Duff HJ, Solylo MA, Mitchell LB. Precordial QT interval dispersion as a marker of torsade de pointes. Disparate effects of class Ia antiarrhythmic drugs and amiodarone. Circulation 1992;86:1376–1382.
Higham PD, Hilton CJ, Aitcheson JD, Furniss SS, Bourke JP, Campbell RWF. Does QT dispersion reflect dispersion of ventricular recovery? (Abstract). Circulation 1992;86:I-392.
Han J, Moe GK. Nonuniform recovery of excitability in ventricular muscle. Circ Res 1964;14:44–60.
Wang SH, Seltz PJ, Liem LB, et al. Can ventricular vulnerability be predicted by dispersion of the QT interval in humans? (Abstract). J Am Coll Cardiol 1997;29(Suppl A):184A
Rosen MR, Jeck CD, Steinberg SF. Autonomic modulation of cellular repolarization and of the electrocardiographic QT interval. J Cardiovasc Electrophysiol 1992;3:487–499.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tan, H.L., Hou, C.J.Y. & Sung, R.J. Effects of Adenosine A1-receptor activation on Torsade de Pointes in Rabbits. Cardiovasc Drugs Ther 13, 441–447 (1999). https://doi.org/10.1023/A:1007812224862
Issue Date:
DOI: https://doi.org/10.1023/A:1007812224862