Abstract
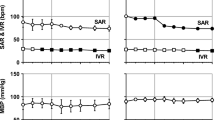
The antifibrillatory effect of pilsicainide, a sodium channel blocker with slow recovery kinetics, was investigated in a canine model of atrial fibrillation. Prolonging the atrial effective refractory period is an important mechanism for pharmacological termination of atrial fibrillation. However, the effectiveness of potassium channel blockers has been questioned because of their reverse-use–dependent property. In eight open-chest dogs, the duration of the atrial endocardial monophasic action potential and the atrial effective refractory period were determined using a Franz catheter. Conduction velocity was obtained from a 96-channel mapping electrode at multiple cycle lengths. Inducibility of sustained atrial fibrillation (>30 minutes) was confirmed by atrial burst pacing during bilateral vagal stimulation, and local fibrillation cycle lengths were measured. Five minutes after restarting fibrillation, pilsicainide (0.6 mg/kg + 0.04 mg/kg/min) was administered. After fibrillation was terminated, measurements were repeated. Pilsicainide successfully terminated atrial fibrillation in 7 of 8 dogs after the median time of 5.1 minutes. The conduction velocity decreased significantly. Although pilsicainide did not affect monophasic action potential duration, it caused use-dependent prolongation of the atrial effective refractory period (P < 0.05), creating postrepolarization refractoriness. Accordingly, pilsicainide prolonged the atrial fibrillation cycle length from 80.6 to 113.8 ms (P < 0.05) before termination of fibrillation. Sodium channel blockers with slow recovery kinetics can prolong the atrial effective refractory period without affecting monophasic action potential duration. Unlike potassium channel blockers, these sodium channel blockers maintain postrepolarization refract
Similar content being viewed by others
References
Herre JM, Scheinman MM. Supraventricular tachycardia. In: Parmley WW, Chatterjee K, Cheitlin MD, Karliner JS, Rapaport E, Scheinman MM, eds. Cardiology: Physiology, Pharmacology, Diagnosis. Philadelphia: J.B. Lippincott, 1988:1-19.
Kannel WE, Abbott RD, Savage DD, McNamara PM. Epidemiologic features of chronic atrial fibrillation: the Framingham Study. N Engl J Med 1982; 308:1018-1022.
Moe GK, Abildskov JA. Atrial fibrillation as a self-sustaining arrhythmia independent of focal discharge. Am Heart J 1959;58:59-70.
Moe GK. On the multiple wavelet hypothesis of atrial fibrillation. Arch Int Pharmacodyn 1962;140:183-188.
Moe GK, Rheinboldt WC, Abildskov JA. A computer model of atrial fibrillation. Am Heart J 1964;67:200-220.
Allessie MA, Lammers WJEP, Bonke FIM, Hollen J. Experimental evaluation of Moe's multiple wavelet hypothsis of atrial fibrillation. In: Zipes DP, Jalife J, eds. Cardiac Electrophysiology and Arrhythmias. New York: Grune & Stratton, 1985:265-274.
Suttorp MJ, Kingma JH, Lie-A-Huen L, Mast EG. Intravnous flecainide versus verapamil for acute conversion of paroxysmal atrial fibrillation or flutter to sinus rhythm. Am J Cardiol 1989;70:693-696.
Fresco C, Proclemer A, Pavan A, et al. Intravenous propafenone in paroxysmal atrial fibrillation: A randomized, placebo-controlled, double-blind, multicenter clinical trial. Paroxysmal Atrial Fibrillation Italian Trial (PAFIT)-2 Investigators. Clin Cardiol 1996;19:409-412.
Miyano S, Sumoto K, Satoh F, et al. New antiarrhythmic agents. N-aryl-8-pyrrolizidinealkanamides. J Med Chem 1985;28:714-717.
Hattori Y, Inomata N, Aisaka K, Ishihara T. Electrophysiological action of N-(2,6-dimethylphenyl)-8-pyrrolizidineacetamide hydrochloride hemihydrate (SUN1165), a new antiarrhythmic agent. J Cardiovasc Pharmacol 1986;8: 998-1002.
Inomata N, Ishihara T, Akaike T. SUN1165, a new antiarrhythmic Na current blocker in ventricular myocytes of guinea pig. Comp Biochem Physiol 1987;87C:237-243.
Wang Z, Page P, Nattel S. The mechanism of flecainide's antiarrhythmic action in experimental atrial fibrillation. Circ Res 1992;71:271-287.
Atarashi H, Inoue H, Hiejima K, Hayakawa H. Conversion of recent-onset atrial fibrillation by a single oral dose of pilsicainide (Pilsicainide Suppression Trial on Atrial Fibrillation). Am J Cardiol 1996;78:694-697.
Kirchhof C, Wijffels M, Brugada J, Planellas J, Allessie M. Mode of action of a new class Ic drug (Org7797) against atrial fibrillation in conscious dogs. J Cardiovasc Pharmacol 1991;17:116-124.
Wang J, Bourne GW, Wang Z, Villemaire C, Talajic M, Nattel S. Comparative mechanisms of antiarrhythmic drug action atrial fibrillation. The importance of use-dependent effects on refractoriness. Circulation 1993;88:1030-1044.
Whalley DW, Grant AO. Role of sodium and potassium channels in atrial electrogenesis and their blockade by antiarrhythmic drugs. In: DiMarco JP, Prystowsky EN, eds. Atrial Arrhythmias: State of the Arts. Armonk, NY: Futura Publishing, 1995:171-200.
Roden DM. Treatment of cardivascular disorders: Arrhythmias. In: Melmon KL, Morrelli HF, Hoffman BB, Nierenberg DW, eds. Clinical Pharmacology: Basic Principles in Therapeutics. New York: McGraw-Hill, 1992:151-185.
Rosen MR. Effect of pharmacological agents on mechanisms responsible for reentry. In: Kulbertus HE, ed. Reentrant Arrhythmias: Mechanisms and Treatment. Baltimore, MD: University Park Press, 1976:283-294.
Nakaya Y-Nii H, Nomura M, Fujino K, Mori H. Effects of lidocaine and quinidine on post-repolarization refractoriness after the basic and premature action potentials: Consideration of aim of antiarrhythmic drug therapy. Am Heart J 1989;118:907-912.
Nakaya Y, Elharrar V, Surawicz B. Effect of mexiletine, amiodarone and disopyramide on the excitability and refractoriness of canine cardiac fibers: Possible relation to antiarrhythmic drug action and classification. Cardiovasc Drugs Ther 1987;1:141-153.
Campbell TJ. Kinetics of onset of rate dependent effects of Class I antiarrhythmic drugs are important in determining their effects on refractoriness in guinea pig ventricle, and provide a theoretical basis for their subclassification. Cardiovasc Res 1983; 17:344-352.
Inoue M, Inoue D, Ishibashi K, et al. Effect of pilsicainide on the atrial fibrillation threshold in guinea pig atria A comparative study with disopyramide, lidocaine and flecainide. Jpn Heart J 1993;34:301-312.
Hattori Y, Inomata N, Aisaka K, Ishihara T. Electrophysiological action of N-(2,6-dimethylphenyl)-8-pyrrolizidineacetamide hydrochloride hemihydrate (SUN1165), a new antiarrhythmic agent. J Cardiovasc Pharmacol 1986;8: 998-1002.
Weirich J, Hohnloser S, Antoni H. Differential analysis of frequency-dependent effects of antiarrhythmic drugs: Importance of the saturation behavior of frequency-dependent sodium-channel blockade. J Cardiovasc Pharmacol 1992; 20(Suppl. 2):S8-S16.
Costard-Jaeckle A, Liem LB, Franz MR. Frequency-dependent effect of quinidine, mexiletine, and their combination on postrepolarization refractoriness in vivo. J Cardiovasc Pharmacol 1989;14:810-817.
Franz MR, Costard A. Frequency-dependent effects of quinidine on the relationship between action potential duration and refractoriness in the canine heart in situ. Circulation 1988;77:1177-1184.
Hondeghem LM, Katzung BG. Time-and voltage-dependent interaction of antiarrythmic drugs with sodium channels. Biochem Biophys Acta 1977;472:373-98.
Hille B. Local anesthestecs. Hydrophilic and hydrophobic pathways for the drug-receptor reaction. J Gen Physiol 1977;69:497-515.
Inomata N, Ohno T, Ishihara T, Akaike N. Antiarrhythmic agents act differently on the activation phase of the Ach-response in guinea-pig atrial myocytes. Br J Pharmacol 1993;108:111-115.
Jackman WM, Friday KJ, Anderson JL, Aliot EM, Clark M, Lazzara R. The long QT syndromes: A critical review, new clinical observations and a unifying hypothesis. Prog Cardiovasc Dis 1988;31:115-172.
Ellenbogen KA, Clemo HF, Stambler BS, Wood MA, Van der Lugt JT. Efficacy of ibutilide for termination of atrial figrillation and flutter. Am J Cardiol 1996; 78:42-45.
Kowey PR, VanderLugt JT, Luderer JR. Safety and risk/benefit analysis of ibutilide for acute conversion of atrial fibrillation/flutter. Am J Cardiol 1996;78:46-52.
Falk RH, Pollak A, Singh SN, Friedrich T. Intravenous dofetilide, a class III antiarrhythmic agent, for the termination of sustained atrial fibrillation or flutter. J Am Coll Cardiol 1997;29:385-390.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kanki, H., Mitamura, H., Takatsuki, S. et al. Postrepolarization Refractoriness as a Potential Anti–Atrial Fibrillation Mechanism of Pilsicainide, a Pure Sodium Channel Blocker with Slow Recovery Kinetics. Cardiovasc Drugs Ther 12, 475–482 (1998). https://doi.org/10.1023/A:1007758217189
Issue Date:
DOI: https://doi.org/10.1023/A:1007758217189