Abstract
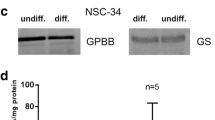
Of the three isozymes of glycogen phosphorylase (GP) known, the brain (B) and muscle (M) isoforms have been reported to occur in brain. We investigated the regional and cellular occurence of the three isozymes in various parts of the rat nervous system, fetal brain and astroglia-rich primary cultures by means of electrophoresis of native proteins with subsequent activity stain and by reverse transcriptase polymerase chain reaction. In the cortex, cerebellum, olfactory bulb, brainstem, spinal cord and dorsal root ganglia, both mRNA and enzyme protein were found for the B and M isozymes. In addition, the liver (L) isoform mRNA was detected in fetal brain and cultured astrocytes. Our studies indicate that there is no regional difference in distribution pattern between brain regions, spinal cord and dorsal root ganglia. In immature brain and cultured glial cells, the additional presence of the L isozyme is possible. These results support the idea that astrocytes express two or even three GP isozymes simultaneously.
Similar content being viewed by others
REFERENCES
Henion, H. F. and Sutherland, E. W. 1957. Immunological differences of phosphorylases. J. Biol. Chem. 224:477–488.
Schane, H. P. 1965. Molecular weight estimation of rat uterine phosphorylase. Anal. Biochem. 11:371–394.
Schliselfeld, L. H., Davis, C. H., and Krebs, E. G. 1970. A comparison of phosphorylase isozymes in the rabbit. Biochemistry 9:4959–4965.
Fletterick, R. J., Burke, J. A., Hwang, P. K., Nakono, K., and Newgard, C. B. 1986. Structural relationships in glycogen phosphorylases. Ann. NY Acad. Sci. 478:220–232.
Newgard, C. B., Hwang, P. K., and Fletterick, R. J. 1989. The family of glycogen phosphorylases: structure and function. Crit. Rev. Biochem. Mol. Biol. 24:69–99.
Newgard, C. B., Littman, D. R., van Genderen, C., Smith, M., and Fletterick, R. J. 1988. Human brain glycogen phosphorylase. Cloning, sequence analysis, chromosomal mapping, tissue expression and comparison with the human liver and muscle isozymes. J. Biol. Chem. 263:3850–3857.
Takeo, K. and Nakamura, S. 1972. Dissociation constants of glucan phosphorylases of rabbit tissue studied by polyacrylamide gel disc electrophoresis. Arch. Biochem. Biophys. 153:1–7.
Sato, K., Morris, H. P., and Weinhouse, S. 1972. Phosphorylase: A new isozyme in rat hepatic tumors and fetal liver. Science 178:879–881.
Richter, F., Böhme, H.-J., and Hofmann, E. 1983. Developmental changes of glycogen phosphorylase b isozymes in rat tissues. Biomed. Biochim. Acta 42:1229–1235.
David, E. S. and Crerar, M. M. 1986. Quantitation of muscle glycogen phosphorylase mRNA and enzyme amounts in adult rat tissues. Biochim. Biophys. Acta 880:78–90.
Mayer, D. and Letsch, J. 1991. Resolution of glycogen phosphorylase isozymes in precast PhastSystem polyacrylamide gels. Electrophoresis 12:297–302.
Mayer, D., Seelmann-Eggebert, G., and Letsch, J. 1992. Glycogen phosphorylase isozymes from hepatoma 3924A and from a non-tumorigenic liver cell line. Biochem. J. 282:665–673.
Sato, K. and Weinhouse, S. 1973. Purification and characterization of the Novikoff hepatoma glycogen phosphorylase and ist relations to the fetal form. Arch. Biochem. Biophys. 159:151–159.
Sato, K., Satoh, K., Imai, F., and Morris, H. P. 1976. Isozyme pattern of glycogen phosphorylase in rat tissues and transplantable hepatomas. Cancer Res. 36:478–495.
Takashi, M., Koshikawa, T., Kurobe, N., and Kato, K. 1989. Elevated concentrations of brain type glycogen phosphorylase in renal cell carcinoma. Jpn. J. Cancer Res. 80:975–980.
Uno, K., Shimada, S., Tsuruta, J., Matsuzaki, H., Tashima, S., and Ogawa, M. 1998. Nuclear localization of brain-type glycogen phosphorylase in some gastrointestinal carcinoma. Histochem. J. 30:553–559.
Gorin, F. A., Mullinax, R. L., Ignacio, P. C., Neve, R. L., and Kurnit, D. M. 1987. McArdle's and Hers' diseases: glycogen phoshorylase transcriptional expression in human tissues. J. Neurogen. 4:293–308.
Gelinas, R. P., Froman, B. E., Mc Elroy, F., Tait, R. C., and Gorin, F. A. 1989. Human brain glycogen phosphorylase: characterization of fetal cDNA and genomic sequences. Mol. Brain Res. 6:177–185.
Reinhart, P. H., Pfeiffer, B., Spengler, S., and Hamprecht, B. 1990. Purification of glycogen phosphorylase from bovine brain and immunocytochemical examination of rat primary cultures using monoclonal antibodies raised against the enzyme. J. Neurochem. 54:1474–1483.
Pfeiffer, B., Elmer, K., Roggendorf, W., and Hamprecht, B. 1990. Immunocytochemical demonstration of glycogen phosphorylase in rat brain slices. Histochemistry 94:73–80.
Pfeiffer, B., Meyermann, R., and Hamprecht, B. 1992. Immunohistochemical co-localization of glycogen phosphorylase with the astroglial markers glial fibrillary acidic protein and S-100 protein in rat brain sections. Histochemistry 97:405–412.
Pfeiffer, B., Buse, E., Meyermann, R., Rocha, M. J. A., and Hamprecht, B. 1993. Glycogen phosphorylase activity and immunoreactivity during pre-and postnatal development of rat brain. Histochemistry 100:265–270.
Pfeiffer, B., Buse, E., Meyermann, R., and Hamprecht, B. 1995. Immunocytochemical localization of glycogen phosphorylase in primary sensory ganglia of the peripheral nervous system of the rat. Histochemistry 103:69–74.
Hamprecht, B. and Löffler, F. 1985. Primary glial cultures as a model for studying hormone action. Meth. Enzymol. 109:341–345.
Bradford, M. M. 1976. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248–254.
Chomczynski, P. and Sacchi, N. 1987. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenolchloroform extraction. Anal. Biochem. 162:156–159.
Sambrook, J., Fritsch, E. F., and Maniatis, T. 1973. Molecular Cloning: A laboratory Manual. Cold Spring Harbor Laboratory Press. Cold Spring Harbor, NY.
Schiebel, K., Pekel, E., and Meyer, D. 1992. The nucleotide sequence of rat liver glycogen phosphorylase cDNA. Biochim. Biophys. Acta 1130:349–351.
Swanson, R. A. 1992. Physiologic coupling of glial glycogen metabolism to neural activity in brain. Can. J. Physiol. Pharmacol. 70:S138-S144.
Dringen, R., Gebhardt, R., and Hamprecht, B. 1993. Glycogen in astrocytes: possible role as lactate supply for neighboring cells. Brain Res. 623:208–214.
Swanson, R. A. and Choi, D. W. 1993. Glial glycogen stores affect neuronal survival during glucose deprivation in vitro. J. Cereb. Blood Flow Metab. 13:162–169.
Ransom, B. R. and Fern, R. 1997. Does astrocytic glycogen benefit axon function and survival in CNS white matter during glucose deprivation? Glia 21:134–141.
Wosilait, D. E. and Sutherland, E. W. 1956. The relationship of epinephrine and glycogen to liver phosphorylase. II. Enzymatic inactivation of liver phosphorylase. J. Biol. Chem. 218:469–481.
Appleman, M. M., Krebs, E. G., and Fischer, E. H. 1966. Purification and properties of inactive liver phosphorylase. Biochemistry 5:2101–2107.
Stalmans, W. and Hers, H. G. 1975. The stimulation of liver phosphorylase b by AMP, fluoride and sulfate. Eur. J. Biochem. 54:341–350.
Tan, A. W. and Nuttall, F. Q. 1975. Characteristics of the dephosphorylated form of phosphorylase purified from rat liver and measurement of its activity in crude liver preparations. Biochem. Biophys. Acta 410:45–60.
Ignacio, P. C., Baldwin, B. A., Vijayan, V. K., Tait, R. C., and Gorin, F. A. 1990. Brain isozyme of glycogen phosphorylase: immunohistochemical localization within the central nervous system. Brain Res. 529:42–49.
Kato, K., Shimizu, A., Kurobe, N., Takashi, M., and Koshikawa, T. 1989. Human brain type glycogen phosphorylase: Quantitative localization in human tissues determined with an immunoassay system. J. Neurochem. 52:1425–1432.
Nihira, M., Anderson, K., Gorin, F. A., and Burns, M. S. 1995. Primate rod and cone photoreceptors may differ in glucose accessibility. Invest. Ophthal. Vis. Sci. 36:1259–1270.
Crerar, M. M., Karlsson, O., Fletterick, R. J., and Hwang, P. K. 1995. Chimeric muscle and brain glycogen phosphorylases define protein domains governing isozyme-specific responses to allosteric activation. J. Biol. Chem. 270:13748–13756.
Lowry, O. H., Schulz, D. W., and Passonneau, J. V. 1967. The kinetics of glycogen phosphorylases from brain and muscle. J. Biol. Chem. 242:271–280.
Guenard, D., Morange, M., and Bue, H. 1977. Comparative study of the effect of 5?-AMP and ist analogs on rabbit glycogen phosphorylase b isozymes. Eur. J. Biochem. 76:447–452.
Sorg, O. and Magistretti, P. J. 1991. Characterization of the glycogenolysis elicited by vasoactive intestinal peptide, noradrenaline and adenosine in primary cultures of mouse cerebral cortical astrocytes. Brain Res. 563:227–233.
Sorg, O. and Magistretti, P. J. 1992. Vasoactive intestinal peptide and noradrenaline exert long-term control on glycogen levels in astrocytes: Blockade by protein synthesis inhibition. J. Neurosci. 12:4923–4931.
Cardinaux, J.-R. and Magistretti, P. J. 1996. Vasoactive intestinal peptide, pituitary adenylate cyclase-activating peptide, and noradrenaline induce the transcription factors CCAAT/enhancer binding protein (C/EBP)-? and C/EBP? in mouse cortical astrocytes: involvement in cAMP-regulated glycogen metabolism. J. Neurosci. 16:919–929.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Pfeiffer-Guglielmi, B., Bröer, S., Bröer, A. et al. Isozyme Pattern of Glycogen Phosphorylase in the Rat Nervous System and Rat Astroglia-Rich Primary Cultures: Electrophoretic and Polymerase Chain Reaction Studies. Neurochem Res 25, 1485–1491 (2000). https://doi.org/10.1023/A:1007676109206
Issue Date:
DOI: https://doi.org/10.1023/A:1007676109206