Abstract
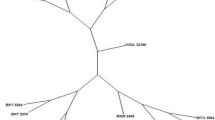
Traditional identification of peach and nectarine varieties relies on the assessment of agronomic traits of the adult plant. This leads to a significant delay of time, constraints to breeders in the surveillance of germplasm and a risk for fruit growers and exporters. We describe a method for rapid assessment of peach and nectarine varieties based on AFLP fingerprinting and extraction of high quality DNA. The best primer pairs were selected from 64 primer combinations that reliably distinguished 8 peach and 6 nectarine varieties. A graphical representation of the detected polymorphisms was shown to simplify the analysis.
Similar content being viewed by others
References
Chandler PM, Robertson M: Gene expression regulated by abscisic acid and its relation to stress tolerance. Annu Rev Plant Physiol Plant Mol Biol 45: 113–141 (1994).
Davies WJ, Jones HG (eds) Drought-induced changes in physiology and ABA. In: Abscisic Acid Physiology and Biochemistry, pp. 63–7
BIOS, Oxford, UK (1991).
Ellis RE, Yuan J, Horvitz HR: Mechanisms and functions of cell death. Annu Rev Cell Biol 7: 663–698 (1991).
Gavrieli Y, Sherman Y, Ben-Sasson SA: Identification of programmed cell death in situ via specific labelling of nuclear DNA fragmentation. J Cell Biol 119: 493–501 (1992).
Goldberg RB, Beals TP, Sanders PM: Anther development: basic principles and practical applications. Plant Cell 5: 1217–1229 (1993).
Havel L, Durzan DJ: Apoptosis during diploid parthenogenesis and early somatic embryogenesis of norway spruce. Int J Plant Sci 157 (1): 8–16 (1996).
Heberle-Bors E: Isolated pollen culture in tobacco: plant reproductive development in a nutshell. Sex Plant Reprod 2: 1–10 (1989).
Hoekstra S, van Zijderveld MH, Louwerse JD, Heidekamp F, van der Mark F: Anther and microspore culture of Hordeum vulgare L. cv. Igri. Plant Science 86: 89–96 (1992).
Hoekstra S, van Zijderveld MH, Heidekamp F, van der Mark F: Microspore culture of Hordeum vulgare L.: the influence of density and osmolality. Plant Cell Rep 12: 661–665 (1993).
Hoekstra S, van Bergen S, van Brouwershaven IR, Schilperoort RA, Heidekamp F: The interaction of 2,4-D application and mannitol pre-treatment in anther and microspore culture of Hordeum vulgare L. cv. Igri. J Plant Physiol 148: 696–700 (1996).
Hoekstra S, van Brouwershaven IR, van Bergen S, Schilperoort RA, Wang M: The role of mannitol, calcium and ABA during pre-treatment in barley androgenesis. Plant Sci 126: 211–218 (1997).
Imamura J, Harada H: Effects of abscisic acid and water stress on the embryo and plantlet production in anther culture of Nicotiana tabacum cv. Samsun. Z PflanzenPhysiol 100: 285–289 (1980).
Jones AM, Dangl JL: Logjam at the styx: programmed cell death in plant. Trends Plant Sci 1: 114–119 (1996).
Koes R, Souer E, van Houwelingen A, Mur L, Spelt C, Quattrocchio F, Wing BJ, Oppedijk B, Ahmed C, Maes T, Gerats T, Hoogeveen P, Meesters M, Kloos D, Mol JNM: Targeted gene inactivation in Petunia by PCR based selection of transposon insertion mutants. Proc Natl Acad Sci USA 92: 8149–8153 (1995).
Kiyosue T, Nakajima M, Yamaguchi I, Satoh S, Kamada H, Harada H: Endogenous levels of abscisic acid in embryogenic cells, non-embryogenic cells and somatic embryos of carrot (Daucus carota L.). Biochem Physiol Pfl 188: 343–347 (1992).
Kuo A, Cappelluti S, Cervantes-Cervantes M, Rodriguez M, Bush DS: Okadaic acid, a protein phosphatase inhibitor, blocks calcium changes, gene expression, and cell death induced by gibberellin in wheat aleurone cells. Plant Cell 8: 259–269 (1996).
Mittler R, Lam E: Sacrifice in the face of foes: pathogeninduced programmed cell death in plants. Trends Microbiol 4: 10–15 (1996).
Pennell RI, Lamb C: Programmed cell death in plants. Plant Cell 9: 1157–1168 (1997).
Raff MC: Social controls on cell survival and cell death. Nature 356: 397–400 (1992).
Rajasekaran K, Hein MB, Vasil IK: Endogenous abscisic acid and indole-3-acetic acid and somatic embryogenesis in cultured leaf explants of Pennisetum purpureum Schum Plant Physiol 84: 47–51 (1987).
Rajasekaran K, Hein MB, Davis GC, Garnes MG, Vasil IK: Endogenous growth regulators in leaves and tissue cultures of Pennisetum purpureum Schum J Plant Physiol 130: 13–25 (1987).
Reynolds TL, Crawford RL: Changes in abundance of an abscisic acid responsive, early cysteine labelled metallothionein gene during pollen embryogenesis in bread wheat (Triticum aestivum). Plant Mol Biol 32: 823–829 (1996).
Ryerson DE, Heath MC: Cleavage of nuclear DNA into oligonucleosomal fragments during cell death induced by fungal infection or by abiotic treatments. Plant Cell 8: 393–402 (1996).
Sambrook J, Fritsch EF, Maniatis T: Molecular Cloning: A Laboratory Manual, 2nd ed. Cold Spring Harbour Laborotory Press, Cold Spring, NY (1989)
Sunderland, N., Huang B, Hills GJ: Disposition of pollen in situ and its relevance to anther/pollen culture. J Exp Bot 35: 521–530 (1994).
Vaux DL, Haecker G, Strasser A: An evolutionary perspective on apoptosis. Cell 76: 777–779 (1994).
Wang M, van Duijn B, van der Meulen RM, Heidekamp F: Effect of abscisic acid analogues on intracellular calcium level and gene expression in barley aleurone protoplasts. In: Karssen CM, van Loon LC, Vreugdenhil D (eds) Progress in Plant Growth Regulation, pp. 635–6
Kluwer Academic Press, Dordrecht, Netherlands (1991).
Wang M, Heimovaara-Dijkstra S, van Duijn B: Modulation of germination of embryos isolated from dormant and nondormant barley grains by manipulation of endogenous abscisic acid. Planta 195: 586–592 (1995).
Wang H, Li J, Bostock RM, Gilchrist DG: Apoptosis: A functional paradigm for programmed plant cell death induced by a host-selective phytotoxin and invoked during development. Plant Cell 8: 375–391 (1996).
Wang M, Oppedijk BJ, Lu X, Van Duijn B, Schilperoort RA: Apoptosis in barley aleurone during germination and its inhibition by abscisic acid. Plant Mol Biol 32: 1125–1134 (1996b).
Wyllie AH: Cell death: The significance of apoptosis. Int Rev Cytol 68: 251–306 (1980).
Yeung EC, Meinke DW: Embryogenesis in angiosperms: development of the suspensory. Plant Cell 5: 1371–1381 (1993).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Manubens, A., Lobos, S., Jadue, Y. et al. DNA Isolation and AFLP Fingerprinting of Nectarine and Peach Varieties (Prunus persica). Plant Molecular Biology Reporter 17, 255–267 (1999). https://doi.org/10.1023/A:1007656110444
Issue Date:
DOI: https://doi.org/10.1023/A:1007656110444