Abstract
Purpose. Peptide transporters PEPT1 and PEPT2 differ substantiallyin their substrate affinity and recognition. The aim of this study is todefine the structural domains which influence the functionalcharacteristics of both transporters
Methods. Two kinds of chimeric peptide transporters (PEPT-N1C2and PEPT-N2C1) were constructed, and their functional characteristicswere compared with those of wild-type transporters in stabletransfectants.
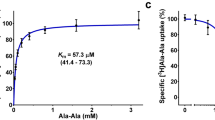
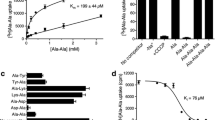
Results. PEPT-N1C2, the N-terminal half of rat PEPT1 and theC-terminal half of rat PEPT2, and the reciprocal chimera PEPT-N2C1were functionally expressed in LLC-PK1 cells. The pH-profiles of [14C]glycylsarcosine uptake by PEPT-N1C2 and PEPT-N2C1 were close tothose of PEPT1 and PEPT2, respectively. Substrate recognition forPEPT-N1C2 and PEPT-N2C1 was also similar to that of PEPT1 andPEPT2, respectively. However, substrate affinities for PEPT-N1C2were higher than those for PEPT1, although those for PEPT-N2C1 andPEPT2 were comparable.
Conclusions. These results indicate that functional regions which areassociated with the extracellular pH changes and are responsible forsubstrate recognition of PEPT1 and PEPT2 may be located in theN-terminal halves of the proteins. In addition, it is suggested that thedomain to affect the substrate affinity exists in the C-terminal as wellas in the N-terminal half of rat PEPT2.
Similar content being viewed by others
REFERENCES
H. Daniel, E. L. Morse, and S. A. Adibi. The high and low affinity transport systems for dipeptides in kidney brush border membrane respond differently to alterations in pH gradient and membrane potential. J. Biol. Chem. 266:19917–19924 (1991).
H. Daniel and S. A. Adibi. Transport of β-lactam antibiotics in kidney brush border membrane. Determinants of their affinity for the oligopeptide/H+ symporter. J. Clin. Invest. 92:2215–2223 (1993).
I. Naasani, K. Sato, K. Iseki, M. Sugawara, M. Kobayashi, and M. Miyazaki. Comparison of the transport characteristics of ceftibuten in rat renal and intestinal brush-border membranes. Biochim. Biophys. Acta 1231:163–168 (1995).
V. Ganapathy and F. H. Leibach. Role of pH gradient and membrane potential in dipeptide transport in intestinal and renal brush-border membrane vesicles from the rabbit. Studies with L-carnosine and glycyl-L-proline. J. Biol. Chem. 258:14189–14192 (1983).
Y.-J. Fei, Y. Kanai, S. Nussberger, V. Ganapathy, F. H. Leibach, M. F. Romero, S. K. Singh, W. F. Boron, and M. A. Hediger. Expression cloning of a mammalian proton-coupled oligopeptide transporter. Nature (Lond.) 368:563–566 (1994).
R. Liang, Y.-J. Fei, P. D. Prasad, S. Ramamoorthy, H. Han, T. L. Feng, M. A. Hediger, V. Ganapathy, and F. H. Leibach. Human intestinal H+ /peptide cotransporter. Cloning, functional expression, and chromosomal localization. J. Biol. Chem. 270:6456–6463 (1995).
H. Saito, M. Okuda, T. Terada, S. Sasaki, and K. Inui. Cloning and characterization of a rat H+/peptide cotransporter mediating absorption of β-lactam antibiotics in the intestine and kidney. J. Pharmacol. Exp. Ther. 275:1631–1637 (1995).
K. Miyamoto, T. Shiraga, K. Morita, H. Yamamoto, H. Haga, Y. Taketani, I. Tamai, Y. Sai, A. Tsuji, and E. Takeda. Sequence, tissue distribution and developmental changes in rat inetestinal oligopeptide transporter. Biochim. Biophys. Acta 1305:34–38 (1996).
M. Boll, M. Herget, M. Wagener, W. M. Weber, D. Markovich, J. Biber, W. Clauss, H. Murer, and H. Daniel. Expression cloning and functional characterization of the kidney cortex high-affinity proton-coupled peptide transporter. Proc. Natl. Acad. Sci. U.S.A. 93:284–289 (1996).
W. Liu, R. Liang, S. Ramamoorthy, Y.-J. Fei, M. E. Ganapathy, M. A. Hediger, V. Ganapathy, and F. H. Leibach. Molecular cloning of PEPT2, a new member of the H+/peptide cotransporter family, from human kidney. Biochim. Biophys. Acta 1235:461–466 (1995).
H. Saito, T. Terada, M. Okuda, S. Sasaki, and K. Inui. Molecular cloning and tissue distribution of rat peptide transporter PEPT2. Biochim. Biophys. Acta 1280:173–177 (1996).
H. Ogihara, H. Saito, B.-C. Shin, T. Terada, S. Takenoshita, Y. K. Inui, and K. Takata. Immunolocalization of H+/peptide cotransporter in rat digestive tract. Biochem. Biophys. Res. Commun. 220:848–852 (1996).
S. Ramamoorthy, W. Liu, Y.-Y. Ma, T. L. Yang-Feng, V. Ganapathy, and F. H. Leibach. Proton/peptide cotransporter (PEPT2) from human kidney: Functional characterization and chromosomal localization. Biochim. Biophys. Acta 1240:1–4 (1995).
M. E. Ganapathy, M. Brandsch, P. D. Prasad, V. Ganapathy, and F. H. Leibach. Differential recognition of β-lactam antibiotics by intestinal and renal peptide transporters, PEPT1 and PEPT2. J. Biol. Chem. 270:25672–25677 (1995).
T. Terada, H. Saito, M. Mukai, and K. Inui. Recognition of β-lactam antibiotics by rat peptide transporters, PEPT1 and PEPT2, in LLC-PK1 cells. Am. J. Physiol. 273, F706–F711 (1997).
T. Terada, H. Saito, M. Mukai, and K. Inui. Characterization of stably transfected kidney epithelial cell line expressing rat H+/peptide cotransporter PEPTI: Localization of PEPT1 and transport of β-lactam antibiotics. J. Pharmacol. Exp. Ther. 281:1415–1421 (1997).
M. M. Bradford. A rapid and sensitive method for the quantitation 23. of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248–254 (1976).
T. Terada, H. Saito, M. Mukai, and K. Inui. Identification of the histidine residues involved in substrate recognition by a rat 24. H+/peptide cotransporter, PEPT1. FEBS Lett. 394:196–200 (1996).
Y.-J. Fei, W. Liu, P. D. Prasad, R. Kekuda, T. G. Oblak, V. Ganapathy, and F. H. Leibach. Identification of the histidyl residue obligatory for the catalytic activity of the human H+/peptide cotransporters PEPT1 and PEPT2. Biochemistry 36:452–460 (1997).
T. Terada, H. Saito, and K. Inui. Interaction of β-lactam antibiotics with histidine residue of rat H+/peptide cotransporters, PEPT1 and PEPT2. J. Biol. Chem. 273:5582–5585 (1998).
A. K. Yeung, S. K. Basu, S. K. Wu, C. Chu, C. T. Okamoto, S. F. Hamm-Alvarez, H. von Grafenstein, W.-C. Shen, K.-J. Kim, M. B. Bolger, I. S. Haworth, D. K. Ann, and V. H. L. Lee. Molecular identification of a role for tyrosine 167 in the function of the human intestinal proton-coupled dipeptide transporter (hPepT1). Biochem. Biophys. Res. Commun. 250:103–107 (1998).
A. M. Pajor, N. Sun, L. Bai, D. Markovich, and P. Sule. The substrate recognition domain in the Na+/dicarboxylate and Na+/sulfate cotransporters is located in the carboxy-terminal portion of the protein. Biochim. Biophys. Acta 1370:98–106 (1998).
F. Döring, D. Dorn, U. Bachfischer, S. Amasheh, M. Herget, and H. Daniel. Functional analysis of a chimeric mammalian peptide transporter derived from the intestinal and renal isoforms. J. Physiol. (Lond) 497:773–779 (1996).
Y.-J. Fei, J.-C. Liu, T. Fujita, R. Liang, V. Ganapathy, and F. H. Leibach. Identification of a potential substrate binding domain in the mammalian peptide transporters PEPT1 and PEPT2 using PEPT1-PEPT2 and PEPT2-PEPT1 chimeras. Biochem. Biophys. Res. Commun. 246:39–44 (1998).
K. J. Buck and S. G. Amara. Chimeric dopamine-norepinephrine transporters delineate structural domains influencing selectivity for catecholamines and 1-methyl-4-phenylpyridinium. Proc. Natl. Acad. Sci. U.S.A. 91:12584–12588 (1994).
D. Peter, T. Vu, and R. H. Edwards. Chimeric vesicular monoamine transporters identify structural domains that influence substrate affinity and sensitivity to tetrabenazine. J. Biol. Chem. 271:2979–2986 (1996).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Terada, T., Saito, H., Sawada, K. et al. N-terminal Halves of Rat H+/Peptide Transporters Are Responsible for Their Substrate Recognition. Pharm Res 17, 15–20 (2000). https://doi.org/10.1023/A:1007554105597
Issue Date:
DOI: https://doi.org/10.1023/A:1007554105597