Abstract
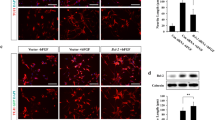
SFME cells are brain-derived neural precursor cells that are acutely dependent on epidermal growth factor (EGF) for survival, undergoing apoptosis within 24 h after EGF withdrawal. Because the expression of the protooncogene bcl-2 inhibits apoptosis induced by the withdrawal of interleukins or nerve growth factor in some growth factor-dependent haematopoietic or neuronal cell cultures, we examined the effect of Bcl-2 expression on cell death of SFME cells in the absence of EGF. SFME cells expressing human Bcl-2 showed prolonged survival when deprived of EGF compared to control cells not expressing Bcl-2. A significant fraction of Bcl-2-expressing cells remained viable for 4 days in the absence of EGF and resumed proliferation upon readdition of EGF to the cultures. These results suggest that apoptosis induced by EGF withdrawal in SFME cells may share common mechanisms with other growth factor-related apoptotic systems.
Similar content being viewed by others
References
Antonsson B, Conti F, Ciavatta A et al. Inhibition of Bax channel-forming activity by Bcl-2. Science. 1997;277:370–2.
Fernandez SM, Bischoff JR. Bcl-2 associates with the rasrelated protein R-ras p23. Nature. 1993;366:274–5.
Garcia I, Martinou I, Tsujimoto Y, Martinou J-C. Prevention of programmed cell death of sympathetic neurons by the bcl-2 proto-oncogene. Science. 1992;258:302–4.
Greenlund LJ, Korsmeyer SJ, Johnson EM. Role of BCL-2 in the survival and function of developing and mature sympathetic neurons. Neuron. 1995;15:649–61.
Hockenberry DM, Oltval Z, Yin X-M, Milliman CL, Korsmeyer SJ. Bcl-2 functions in an antioxidant pathway to prevent apoptosis. Cell. 1993;75:241–51.
Kremer NE, D'Arcangelo G, Thomas SM, DeMarco M, Brugge JS, Halegoua S. Signal transduction by nerve growth factor and fibroblast growth factor in PCI2 cells requires a sequence of Src and Ras actions. J Cell Biol. 1991;115:809–19.
Lendahl U, Zimmerman LB, McKay RDG. CNS stem cells express a new class of intermediate filament protein. Cell. 1990;60:585–95.
Loo DT, Fuquay JI, Rawson CL, Barnes DW. Extended culture of mouse embryo cells without senescence: inhibition by serum. Science. 1987;236:200–2.
Loo D, Rawson C, Ernst T, Shirahata S, Barnes D. Primary and multipassage culture of mouse embryo cells in serum-containing and serum-free milk. In: Baserga R, ed. Cell growth and division: a practical approach. Oxford: IRL Press; 1989:17–35.
Loo DT, Sakai Y, Rawson CL, Barnes DW. Serial passage of embryonic human astrocytes in serum-free, hormone-supplemented medium. J Neurosci Res. 1991;28:101–9.
Loo DT, Althoen MC, Cotman CW. Down regulation of nestin by TGF-B or serum in SFME cells accompanies differentiation into astrocytes. Neuroreport. 1994;5:1–5.
Loo DT, Althoen MC, Cotman CW. Differentiation of SFME cells into astrocytes is accompanied by induction of glutamine synthetase activity. J Neurosci Res. 1995;42:184–91.
Martinou JC, Dubois-Dauphin M, Staple JK et al. Overexpression of BCL-2 in transgenic mice protects neurons from naturally occurring cell death and experimental ischemia. Neuron. 1994;13:1017–30.
Merry DE, Veis DJ, Hickey WF, Korsmeyer SJ. Bcl-2 protein expression is widespread in the developing nervous system and retained in the adult PNS. Development. 1994;120:301–11.
Michaelidis TM, Sendtner M, Cooper JD et al. Inactivation of bcl-2 results in progressive degeneration of motoneurons, sympathetic and sensory neurons during early postnatal development. Neuron. 1996;17:75–89.
Minn AJ, Velez P, Schendel SL et al. Bcl-x(L) forms an ion channel in synthetic lipid membranes. Nature. 1997;385: 353–7.
Nishiyama K, Collodi P, Barnes D. Regulation of glial fibrillary acidic protein in serum-free mouse embryo (SFME) cells by leukemia inhibitory factor and related peptides. Neurosci Lett. 1993;163:114–6.
Oppenheim RW. Cell death during development of the nervous system. Annu Rev Neurosci. 1991;14:453–501.
Rawson C, Smith C, Barnes D. Death of serum-free mouse embryo cells caused by epidermal growth factor deprivation is prevented by cycloheximide, 12-O-tetradecanoylphorbol-13-acetate, or vanadate. Exp Cell Res. 1990;186:177–81.
Rawson CL, Loo DT, Duimstra JR, Hedstrom OR, Schmidt EE, Barnes DW. Death of serum-free mouse embryo cells caused by epidermal growth factor deprivation. J Cell Biol. 1991;113:671–80.
Reed JC. Bcl-2 and the regulation of programmed cell death. J Cell Biol. 1994;124:1–6.
Rozakis-Adcock M, Fernley R, Wade J, Pawson T, Botwell D. The SH2 and SH3 domains of mammalian Grb2 couple the EGF receptor to the Ras activator msosl. Nature. 1993;363: 83–5.
Sakai Y, Rawson C, Lindburg K, Barnes D. Serum and transforming growth factor B regulate glial fibrillary acidic protein in serum-free-derived mouse embryo cells. Proc Natl Acad Sci USA. 1990;87:8378–82.
Satoh T, Nakafuku M, Miyajima A, Kaziro Y. Involvement of ras p21 protein in signal-transduction pathways from interleukin 2, interleukin 3, and granulocyte/macrophage colony-stimulating factor, but not from interleukin 4. Proc Natl Acad Sci USA. 1991;88:3314–8.
Shirahata S, Rawson C, Loo D, Chang YJ, Barnes D. Ras and neu oncogenes reverse serum inhibition and epidermal growth factor dependence of serum-free mouse embryo cells. J Cell Physiol. 1990;144:69–76.
Solem M, Rawson C, Lindburg K, Barnes D. Transforming growth factor beta regulates cystatin C in serum-free mouse embryo (SFME) cells. Biochem Biophys Res Commun. 1990;172:945–51.
Vaux DL, Cory S, Adams JM. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature. 1988;335:440–2.
Wang HG, Miyashita T, Takayama S et al. Apoptosis regulation by interaction of Bcl-2 protein and Raf-I kinase. Oncogene. 1994;9:2751–6.
Wang HG, Rapp UR, Reed JC. Bcl-2 targets the protein kinase Raf-1 to mitochondria. Cell. 1996;87:629–38.
Weisz PV, Solem M, Barnes D. Expression of a TGF beta regulated, brain-specific MRNA in serum-free mouse embryo (SFME) cells. Neurosci Lett. 1993;154:153–6.
Zha J, Harada H, Yang E, Jocket J, Korsmeyer SJ. Serine phosphorylation of death agonist BAD in response to survival factor results in binding to 14-3-3 not BCL-Xl. Cell. 1996;87:619–28.
Zhong LT, Sarafian T, Kane DJ et al. Bcl-2 inhibits death of central neural cells induced by multiple agents. Proc Natl Acad Sci USA. 1993;90:4533–7.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Loo, D., Bradford, S., Helmrich, A. et al. Bcl-2 inhibits cell death of serum-free mouse embryo cells caused by epidermal growth factor deprivation. Cell Biol Toxicol 14, 375–382 (1998). https://doi.org/10.1023/A:1007518909429
Issue Date:
DOI: https://doi.org/10.1023/A:1007518909429