Abstract
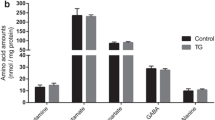
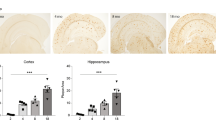
The effect of amyloid β (Aβ), the major constituent of the Alzheimer's (AD) brain on lipid metabolism was investigated in cultured nerve cells and in a fetal rat brain model. Differentiated (NGF) and undifferentiated PC12 cells or primary cerebral cell cultures were incubated with [14C]acetate in the absence or presence of Aβ1–40. Incorporation of label into lipid species was determined after lipid extraction and TLC separation. Phosphatidylcholine (PC) and phosphatidylserine (PS) synthesis was increased by Aβ1–40, in a dose dependent manner, an effect which was more pronounced in differentiated PC12 cells. A significant proportion of radioactivity (5–6%) was released into the medium with a radioactivity distribution similar to that of the cellular lipids. Cholesterol and PC were the highest labeled medium lipids. Increasing Aβ1–40 concentration up to 0.1 μg/ml in cerebral cells but not in PC12 cells, caused a relative increase (1.5 fold) in release of PS, while that of PE decreased. Stimulation of PS release may possibly be associated with apoptotic cell death. Aβ1–40 peptide (5 μg) was administered intraperitonealy into rat fetuses (18 days gestation) along with [14C]acetate (2μCi/fetus). After 24 h, the maternal-fetal blood supply was occluded for 20 min (ischemia) followed by 15 min reperfusion. Fetuses were killed and liver and brain tissue subjected to lipid extraction and radioactivity determination after TLC. Aβ1–40 peptide increased synthesis of different classes of lipids up to 20–40% in brain tissue compared to controls. Labeling of liver lipids was decreased by Aβ1–40 by 20–30%. A general decrease in synthesis of lipids was observed after ischemia/reperfusion. Our data suggest that Aβ1–40 peptide regulates normal lipid biosynthesis but under ischemia it compromises it. The latter finding may confirm the oxidative stress etiology in AD and suggests that Aβ1–40 modulation of lipid metabolism may have Alzheimer's pathological relevance, particularly at high peptide concentrations.
Similar content being viewed by others
REFERENCES
Greenberg, B. and Murphy, M. 1994. Toward an integrated discovery and development program in Alzheimer' disease: The amyloid hypothesis. Neurobiol. Aging 15:S105-109.
Selkoe, D. 1994. Alzheimer' disease: a central role for amyloid. J. Neuropathol. Exp. Neurol. 53:438-447.
Shoji, M., Golde, T., Ghiso, J., Cheung, T., Estus, S., Shaffer, L., Cai, X-D., McKay, D., Tintner, R., Frangione, B., and Younkin, S. 1992. Production of the Alzheimer amyloid β protein by normal proteolytic processing. Science 258:126-129.
Haass, C., Schlossmacher, M., Hung, A., Vigo-Pelfrey, C., Mellon, A., Ostaszewski, B., Lieberburg, I., Koo, E., Schenk, D., Teplow, D., and Selkoe, D. 1992. Amyloid β-peptide is produced by cultured cells during normal metabolism. Nature London 359:322-325.
Seubert, P., Vigo-Pelfrey, C., Esch, F., Lee M., Dovey, H., Davis, D., Sinha, S., Schlossmacher, M., Whaley, J., Swindle-Hurst, C., McCormack, R., Wolfert, R., Selkoe, D., Lieberburg, I., and Schenk, D. 1992. Isolation and quantification of soluble Alzheimer' β-peptide from biological fluids. Nature London 359:325-327.
Koudinov, A., Koudinova, N., Kumar, A., Beavis, R., and Ghiso, J. 1996. Biochemical characterization of Alzheimer' soluble amyloid beta protein in human cerebrospinal fluid: association with high density lipoproteins. Biochem. Biophys. Res. Commun. 223:592-597.
Koudinov, A. R., Berezov, T. T., Kumar, A., and Koudinova, N. V. 1998. Alzheimer' amyloid β interaction with normal human plasma high density lipoprotein: association with apolipoprotein and lipids. Clin. Chim. Acta 270:75-84.
Koudinov, A. R. and Koudinova, N. V. 1997. Soluble amyloid beta protein is secreted by HepG2 cells as an apolipoprotein. Cell Biol. Internat. 25:265-271.
Mahley, R., Innerarity, T., Rail, S., and Weisgraber, K. 1984. Plasma lipoproteins: apolipoprotein structure and function. J. Lipid Res. 25:1277-1294.
Koudinov, A. R., Koudinova, N. V., and Berezov, T. T. 1996. Alzheimer' peptides Aβ1-40 and Aβ1-28 inhibit the plasma cholesterol esterification rate. Biochem. Mol. Biol. Inter. 38:747-752.
Koudinova, N., Berezov, T., and Koudinov, A. 1996. Multiple inhibitory effects of Alzheimer' peptide Aβ1-40 on lipid byosynthesis in HepG2 cells. FEBS Lett. 395:204-206.
Ehmann, W. D., Markesbery, W. R., Alauddin, M., Hossain, T. I., and Brubaker, E. H. 1986. Brain trace elements in Alzheimer' disease. Neurotoxicology 7:197-206.
Prasad M. R., Lovell M. A., Dhillon H., Markesbery W. 1998. Regional membrane phospholipid alterations in Alzheimer' disease. Neurochem Res 23:81-88.
Farooqui, A. A., Wells, K., and Horrocks, L. A. 1995. Breakdown of membrane phospholipids in Alzheimer' diseaseinvolvement of excitatory amino-acid receptors. Mol. Chem. Neuropathol. 25:155-173.
Mason, R. P., Shoemaker, W. J., Shajenko, L., Chambers, T. E., and Herbette, L. G. 1992. Evidence for changes in the Alzheimer' disease brain cortical membrane structure mediated by cholesterol. Neurobiology Aging 13:413-418.
Nithsch, R. M., Blusztajn, J. K., Pittas, A. G., Slack, B. E., and Wurtman, R. J. 1992. Evidence for a membrane defect in Alzheimer disease brain. Proc. Natl. Acad. Sci. USA. 89:1671-1675.
Markesbery, W. R. 1997. Oxidative stress hypothesis in Alzheimer' disease. Free Rad. Biol. Med. 23:134-147.
Balasz, L. and Leon, M. 1994. Evidence of an oxidative challenge in the Alzheimer' brain. Neurochem. Res. 19:1131-1137.
Bazan, N. G. Jr. 1970. Effects of ischemia and electroconvulsive shock on free fatty acid pool in the brain. Biochim. Biophys. Acta. 218:1-10.
Magal, E., Goldin, E., Harel, S., and Yavin, E. 1988. The uteroplacental ischemic rat embryo: A. Lactic acid accumulation and prostaglandin formation in the fetal brain. J. Neurochem. 51:75-80.
Yavin, E. and Marie-Billia, D. 1997. Death in early postmitotic cerebral cortex cells after transient oxygen and glucose deprivation is apoptotic. J. Neurosci. Res. 47:471-478.
Hajra, A. and Radin, N. 1978. Lipid extraction of tissues with a low toxicity solvent. Anal. Biochem. 90:420-426.
Folch, J., Lees, M., and Sloane-Stanley, G. H. 1957. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 226:497-509.
Friedman, L. K., Koudinov, A. R. 1999. Unilateral GluR2(B) hippocampal knockdown: a possible novel partial seizure model in young rat. J. Neurosci. 19, 9412-9425.
Araki, W., Wurtman, R. J. 1997. Control of membrane phosphatidylcholine biosynthesis by diacylglycerol levels in neuronal cells undergoing neurite outgrowth. Proc Natl Acad Sci U S A, 94:11946-11950.
Ulus, I. H, Wurtman, R. J., Mauron, C., Blusztajn, J. K. 1989. Choline increases acetylcholine release and protects against the stimulation-induced decrease in phosphatide levels within membranes of corpus striatum. Brain Res. 484:217-26
Wolfe, L. S. 1982. Eicosanoids: Prostaglandins, thromboxane, leukotrienes and other derivatives of carbon-20 unsaturated fatty acids. J. Neurochem. 38:1-14.
Bazan, N. G. 1990. Involvement of arachidonic acid and platelet-activating factor in the response of the nervous system to ischemia and convulsions. In Lipid mediators in ischemic brain damage and experimental epilepsy. New Trends Lipid Mediators Res. 4:241-252.
Takai, Y., Kishimoto, A., Inoue, M., and Nishizuka, Y. 1977. Studies on a cyclic nucleotide-independent protein kinase and its proenzyme in mammalian tissues. I. Purification and characterization of an active enzyme from bovine cerebellum. J. Biol. Chem. 252:7603-7609.
Routtenberg, A. 1986. Synaptic plasticity and protein kinase C. Prog. Brain Res, 69:411-435.
Kuperstein, F., Koudinova, N., and Yavin, E. 1998. Regulation of PKC and MAPK-related phosphorylation cascades by β amyloid 1-40 during oxygen deprivation in rat cerebral neurons. Neurosci Lett. 51, S24.
Maulik, N., Kagan, V. E., Tyurin, V. A., and Das, D. K. 1998. Redistribution of phosphatidylethanolamine and phosphatidylserine precedes reperfusion-induced apoptosis. Am. J. Physiol. 274:H242-248.
Urmoneit, B., Prikulis, I., Wihl, G., Durso, D., Frank, R., Heeren, J., Belsiegel, U., Prior, R. 1997. Cerebrovascular smooth muscle cells internalize Alzheimer amyloid beta protein via a lipoprotein pathway: Implications for cerebral amyloid angiopathy. Lab Investig. 77:157-166.
Holtzman, D. M., Bales, K. R., Wu, S., Bhat, P., Parsadanian, M., Fagan, A. M., Chang, L. K., Sun, Y. L., Paul, S. M. 1999. Expression of human apolipoprotein E reduces amyloid-beta deposition in a mouse model of Alzheimer' disease J. Clin. Investig. 103:R15-R21.
Glozman, S., and Yavin, E. 1997. Lipid peroxides are generated by fetal rat brain after episodes of global ischemia in utero. Neurochem. Res. 22:201-208.
Hophy, M., Harel, S., and Yavin, E. 1996. Prostacyclin accumulates in a rat model of uteroplacental insufficiency: Implications for intrauterine growth retardation. Reprod. Fertil. Dev. 8:895-901.
Ames, B. N., Shigenage, M. K., and Hagen, T. M. 1993. Oxidants, antioxidants and the degenerative diseases of aging. Proc Natl Acad Sci USA. 90:7915-7922.
Smith, M. A., Hirai, K., Hsiao, K., Pappola, M., and Perry, G. 1998. Amyloid-β deposition in Alzheimer transgenic mice is associated with oxidative stress. J. Neurochem. 70:2212-2215.
Gurney, M. E., Chiu, A. Y., Dal Canto, M. C., Polchow, C. Y. et al. 1994. Motor neuron degeneration in mice that express a human Cu, Sn, superoxide dismutase mutation. Science 264:1772-1775.
Pettegrew, J. W., Moossy, J., Withers, G., McKeag, D., and Panchalingam, K. 1988. [31P] nuclear magnetic resonance study of the brain in Alzheimer' disease. J. Neuropathol. Exp. Neurol. 47:235-248.
Klunk, W. E., Panchalingam, K., McClure, R. J., and Pettegrew, J. W. 1996. Quantitative H-1 and P-31 MRS of PCA extracts of postmortem Alzheimer' disease brain. Neurobiology Aging 17:349-357.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Koudinova, N.V., Koudinov, A.R. & Yavin, E. Alzheimer's Aβ1–40 Peptide Modulates Lipid Synthesis in Neuronal Cultures and Intact Rat Fetal Brain Under Normoxic and Oxidative Stress Conditions. Neurochem Res 25, 653–660 (2000). https://doi.org/10.1023/A:1007511120099
Issue Date:
DOI: https://doi.org/10.1023/A:1007511120099