Abstract
Purpose. Dietary flavonoids have been reported to be potent inhibitorsof drug metabolizing enzymes. In the present study we examined theinducing effect of three of these compounds, chrysin, quercetin andgenistein, on UDP-glucuronosyltransferase (UGT) in the humanintestinal cell line Caco-2.
Methods. The induction of UGT by flavonoid pretreatment was studiedboth in the intact cells and cell homogenates, measured as theglucuronidation of chrysin, and by immunoblot analysis of the UGT 1A protein.
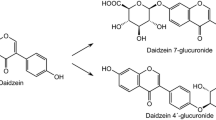
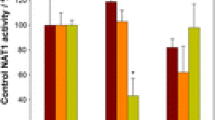
Results. Exposure of Caco-2 cells to 50 μM chrysin resulted in a3.8-fold increase in chrysin glucuronidation in intact cells (p < 0.0001)with a 38% decrease in sulfation (p < 0.01). In the cell homogenatethe induction was much larger, 14-fold. The induction was slow todevelop with maximum induction after 3–4 days. Interestingly, theisoflavonoid genistein was without effect. Immunoblot analysis ofCaco-2 cell microsomes with a UGT1A subfamily-selective antibodyshowed a markedly increased band at about 59 kDa, consistent withinduction of one or more UGT1A isoforms. A 5-week exposure ofCaco-2 cells to low concentrations (10 μM) of chrysin or quercetinalso showed markedly increased glucuronidation activity.
Conclusions. Diet-mediated induction of intestinal UGT may beimportant for the bioavailability of carcinogens and other toxicchemicals as well as therapeutic drugs.
Similar content being viewed by others
REFERENCES
P. Artursson. Epithelial transport of drugs in cell culture. I: a model for studying the passive diffusion of drugs over intestinal absorbtive (Caco-2) cells. J. Pharm. Sci. 79:476–482 (1990).
J. Hunter, M. A. Jepson, T. Tsuruo, N. L. Simmons, and B. H. Hirst. Functional expression of P-glycoprotein in apical membranes of human intestinal Caco-2 cells·kinetics of vinblastine secretion and interaction with modulators. J. Biol. Chem. 268:14991–14997 (1993).
V. Meunier, M. Bourrié, Y. Berger, and G. Fabre. The human intestinal epithelial cell line Caco-2; pharmacological and pharmacokinetic applications. Cell Biol. Toxicol. 11:187–194 (1995).
U. K. Walle, A. Galijatovic, and T. Walle. Transport of the flavonoid chrysin and its conjugated metabolites by the human intestinal cell line Caco-2. Biochem. Pharmacol. 58:431–438 (1999).
T. Prueksaritanont, L. M. Gorham, J. H. Hochman, L. O. Tran, and K. P. Vyas. Comparative studies of drug-metabolizing enzymes in dog, monkey, and human small intestines, and in Caco-2 cells. Drug Metab. Dispos. 24:634–642 (1996).
L.-S. L. Gan and D. R. Thakker. Applications of the Caco-2 model in the design and development of orally active drugs: elucidation of biochemical and physical barriers posed by the intestinal epithelium. Adv. Drug Del. Rev. 23:77–98 (1997).
A. Baranczyk-Kuzma, J. A. Garren, I. J. Hidalgo, and R. T. Borchardt. Substrate specificity and some properties of phenol sulfotransferase from human intestinal Caco-2 cells. Life Sci. 49:1197–1206 (1991).
P. J. Chikhale and R. T. Borchardt. Metabolism of L-α-methyldopa in cultured human intestinal epithelial (Caco-2) cell monolayers: Comparison with metabolism in vivo. Drug Metab. Dispos. 22:592–600 (1994).
S. Bjorge, K. L. Hamelehle, R. Homan, S. E. Rose, D. A. Turluck, and D. S. Wright. Evidence for glucuronide conjugation of p-nitrophenol in the Caco-2 cell model. Pharm. Res. 8:1441–1443 (1991).
R. A. Walgren, U. K. Walle, and T. Walle. Transport of quercetin and its glucosides across human intestinal epithelial Caco-2 cells. Biochem. Pharmacol. 55:1721–1727 (1998).
A. Galijatovic, Y. Otake, U. K. Walle, and T. Walle. Extensive metabolism of the flavonoid chrysin by human Caco-2 and Hep G2 cells. Xenobiotica in press (1999).
X.-Y. Sun, C. A. Plouzek, J. P. Henry, T. T. Y. Wang, and J. M. Phang. Increased UDP-glucuronosyltransferase activity and decreased prostate specific antigen production by biochanin A in prostate cancer cells. Cancer Res. 58:2379–2384 (1998).
M.-H. Siess, J.-P. Mas, M.-C. Canivenc-Lavier, and M. Suschetet. Time course of induction of rat hepatic drug-metabolizing enzyme activities following dietary administration of flavonoids. J. Toxicol. Envir. Health 49:481–496 (1996).
M.-C. Canivenc-Lavier, M.-F. Vernevaut, M. Totis, M.-H. Siess, J. Magdalou, and M. Suschetet. Comparative effects of flavonoids and model inducers on drug-metabolizing enzymes in rat liver. Toxicology 114:19–27 (1996).
O. H. Lowry, N. J. Rosebrough, A. L. Farr, and R. J. Randall. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265–275 (1951).
U. K. Laemmli. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685 (1970).
H. Towbin, T. Staehelin, and J. Gordon. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 76:4350–4354 (1979).
M. G. L. Hertog, P. C. H. Hollman, and M. B. Katan. Content of potentially anticarcinogenic flavonoids of 28 vegetables and 9 fruits commonly consumed in the Netherlands. J. Agric. Food Chem. 40:2379–2383 (1992).
Y.-C. Kao, C. Zhou, M. Sherman, C. A. Laughton, and S. Chen. Molecular basis of the inhibition of human aromatase (estrogen synthetase) by flavone and isoflavone phytoestrogens: a site-directed mutagenesis study. Environ. Health Perspect. 106:85–92 (1998).
J. W. Critchfield, J. E. Coligan, T. M. Folks, and S. T. Butera. Casein kinase II is a selective target of HIV-1 transcriptional inhibitors. Proc. Natl. Acad. Sci USA 94:6110–6115 (1997).
D. W. Boulton, U. K. Walle, and T. Walle. Fate of the flavonoid quercetin in human cell lines: Chemical instability and metabolism. J. Pharm. Pharmacol. 51:353–359 (1999).
E. A. Eaton, U. K. Walle, A. J. Lewis, T. Hudson, A. A. Wilson, and T. Walle. Flavonoids, potent inhibitors of the human P-form phenolsulfotransferase: potential role in drug metabolism and chemoprevention. Drug Metab. Dispos. 24:232–237 (1996).
A. Abid, I. Bouchon, G. Siest, and N. Sabolovic. Glucuronidation the Caco-2 human intestinal cell line: Induction of UDP-glucuronosyltransferase 1*6. Biochem. Pharmacol. 50:557–561 (1995).
P. A. Münzel, G. Bookjans, G. Mehner, T. Lehmköster, and K. W. Bock. Tissue-specific 2,3,7,8-tetrachlorodibenzo-p-dioxin-inducible expression of human UDP-glucuronosyltransferase UGT1A6. Arch. Biochem. Biophys. 335:205–210 (1996).
P. A. Münzel, S. Schmohl, H. Heel, K. Kälberer, B. S. Bock-Hennig, and K. W. Bock. Induction of human UDP glucuronosyltransferases (UGT1A6, UGT1A9, and UGT2B7) by t-butylhydroquinone and 2,3,7,8-tetrachlorodibenzo-p-dioxin in Caco-2 cells. Drug Metab. Dispos. 27:569–573 (1999).
C. D. King, M. D. Green, G. R. Rios, B. L. Coffman, I. S. Owens, W. P. Bishop, and T. R. Tephly. The glucuronidation of exogenous and endogenous compounds by stably expressed rat and human UDP-glucuronosyltransferase 1.1 Arch. Biochem. Biophys. 332:92–100 (1996).
M. D. Green, C. D. King, B. Mojarrabi, P. I. Mackenzie, and T. R. Tephly. Glucuronidation of amines and other xenobiotics catalyzed by expressed human UDP-glucuronosyltransferase 1A3. Drug Metab. Dispos. 26:507–512 (1998).
Z. Cheng, A. Radominska-Pandya, and T. R. Tephly. Cloning and expression of human UDP-glucuronosyltransferase (UGT) 1A8. Arch. Biochem. Biophys. 356:301–305 (1998).
T. Ebner and B. Burchell. Substrate specificities of two stably expressed human liver UDP-glucuronosyltransferases of the UGT1 gene family. Drug Metab. Dispos. 21:50–56 (1993).
M. D. Green, E. M. Oturo, and T. R. Tephly. Stable expression of a human liver UDP-glucuronosyltransferase (UGT2B15) with activity toward steroid and xenobiotic substrates. Drug Metab. Dispos. 22:799–805 (1994).
E. Lévesque, M. Beaulieu, M. D. Green, T. R. Tephly, A. Bélanger, and D. W. Hum. Isolation and characterization of UGT2B15 (Y85): a UDP-glucuronosyltransferase encoded by a polymorphic gene. Pharmacogenetics 7:317–325 (1997).
C. P. Strassburg, N. Nguyen, M. P. Manns, and R. H. Tukey. UDP-Glucuronosyltransferase activity in human liver and colon. Gastroenterology 116:149–160 (1999).
Y. Ishii, A. Takami, K. Tsuruda, A. Kurogi, H. Yamada, and K. Oguri. Induction of two UDP-glucuronosyltransferase isoforms sensitive to phenobarbital that are involved in morphine glucuronidation: production of isoform-selective antipeptide antibodies toward UGT1.1r and UGT2B1. Drug Metab. Dispos. 25:163–167 (1997).
T. Iyanagi, Y. Emi, and S. Ikushiro. Biochemical and molecular aspects of genetic disorders of bilirubin metabolism. Biochem. Biophys. Acta 1407:173–184 (1998).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Galijatovic, A., Walle, U.K. & Walle, T. Induction of UDP-Glucuronosyl-Transferase by the Flavonoids Chrysin and Quercetin in Caco-2 Cells. Pharm Res 17, 21–26 (2000). https://doi.org/10.1023/A:1007506222436
Issue Date:
DOI: https://doi.org/10.1023/A:1007506222436