Abstract
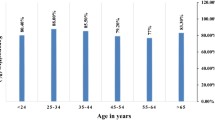
Helicobacter pylori is one of the most common bacterial infections worldwide. To evaluate the prevalence of this infection in Gipuzkoa (Basque Country, Spain) we studied the presence of antibodies against Helicobacter pylori (HPAb) using a second-generation EIA (Cobas Core). The study was performed on two groups of subjects: a middle-class group, 2–78 years-old (n = 1335) and a group of slum dwellers, 2–15 years-old (n = 89). In the middle-class group the prevalence of HPAb in children under 6 was 3.1% (3/96); the prevalence was significantly greater in older compared to younger age groups, reaching 84.3% (102/121) in adults 50–59 years. The geometric mean of the titer in seropositive subjects was also greater in older age groups. By logistic regression analysis the prevalence of HPAb was associated with age, educational level and geographic origin but not with sex, smoking, alcohol consumption, or use of nonsteroid anti-inflammatory drugs. The prevalence of HPAb was much higher in the slum-dwelling group 2–15 years-old (55.5% of children 2– 5 years-old). The results indicate that H. pylori infection was more common in adult people from our geographic region than in those from other developed countries and show that socioeconomically deprived children constitute at present a group at high risk of acquiring infection in our region.
Similar content being viewed by others
References
Warren JR, Marshall B. Unidentified curved bacili on gastric epithelium in active chronic gastritis. Lancet 1983; 1: 1273–1275.
NIH Consensus Development Panel on Helicobacter pylori in Peptic Ulcer Disease. Helicobacter pylori in peptic ulcer disease. JAMA 1994; 272: 65–69.
Parsonnet J. Helicobacter phylori in the stomach — A paradox unmasked. N Engl J Med 1996; 335: 378–280.
Blaser MJ. Helicobacter pylori and the pathogenesis of gastroduodenal inflammation. J Infect Dis 1990; 161: 626–633.
Infection with Helicobacter pylori. In: World Health Organization. IARC monographs on the evaluation of carcinogenic risks to humans. Vol. 61. Schistosomes, liver flukes and Helicobacter pylori. Lyon, France: International Agency for Research on Cancer, 1994: 177–240.
Boixeda D, Gisbert JP, Martin de Argila C, et al. Infección por Helicobacter pylori a nivel bulbar en diferentes diagnósticos endoscópicos. Rev Esp Enf Ap Digest 1995; 195: 220–225.
López-Brea M, Martin E, Alarcó T, et al. Seguimiento de la respuesta serológica cuantitativa al tratamiento de la infección por Helicobacter pylori en niños. Enferm Infecc Microbiol Clin 1993; 11: 33–35.
Instituto Vasco de Estadística. Familias por la situación económica objetiva, apreciación subjetiva y situación económica general, según la zona de residencia (1989). Anuario Estadístico Vasco 1992, pp. 263–264.
García-Bengoechea M, Emparanza JI, Sarriugarte A, et al. Antibodies to hepatitis C virus: A cross-sectional study in patients attending a trauma unit or addmited to hospital for elective surgery. Eur J Gastroenterol Hepatol 1995; 7: 237–241.
Taylor DN, Blaser MJ. The epidemiology of Helicobacter pylori infection. Epidemiol Rev 1991; 13: 42–59.
Mégraud F, Brassens-Rabbé M-P, Denis F, et al. Seroepidemiology of Campylobacter pylori infection in various populations. J Clin Microbiol 1989; 27: 1870–1873.
Graham DY, Malaty HM, Evans DG, et al. Epidemiology of Helicobacter pylori in an asymptomatic population in the United States. Effect of age, race, and socioeconomic status. Gastroenterology 1991; 100: 1495–1501.
The EUROGAST Study Group. Epidemiology of, and risk factors for, Helicobacter pylori infection among 3194 asymptomatic subjects in 17 populations. Gut 1993; 34: 1672–1676.
Bodhidatta L, Hoge CW, Churnratanakul S, et al. Diagnosis of Helicobacter pylori infection in a developing country: Comparison of two ELISAs and a seroprevalence study. J Infect Dis 1993; 168: 1549–1553.
Lindkvist P, Asrat D, Nilsson I, et al. Age at acquisition of Helicobacter pylori infection: Comparison of a high and a low prevalence country. Scand J Infect Dis 1995; 28: 181–184.
Asaka M, Kimura T, Kudo M, et al. Relationship of Helicobacter pylori to serum pepsinogens in an asymptomatic Japanese population. Gastroenterology 1992; 102: 760–766.
Sitas F, Forman D, Yarnell JWG, et al. Helicobacter pylori infection rates in relation to age and social class in a population of Welsh men. Gut 1991; 32: 25–28.
Gasbarrini G, Pretolani S, Bonvicini F, et al. A population based study of Helicobacter pylori infection in a European country: The San Marino Study. Relations with gastrointestinal diseases. Gut 1995; 36: 838–844.
Navarro F, Coll P, Sáinz S, et al. Evaluación de dos preparados comerciales para la detección de anticuerpos especificos de Helicobacter pylori en pacientes sometidos a gastroscopia. Estudio de la seroprevalencia en la población asintomática. Enferm Infecc Microbiol Clin. 1992; 10: 190–194.
Thomas Je, Gibson GR, Darboe MK, et al. Isolation of Helicobacter pylori from human faeces. Lancet 1992; 340: 1194–1195.
Shames B, Krajden S, Fuksa M, et al. Evidence for the occurrence of the same strain of Campylobacter pylori in the stomach and dental plaque. J Clin Microbiol 1989; 27: 2849–2850.
Ferguson DA Jr, Li C, Patel NR, et al. Isolation of Helicobacter pylori from saliva. J Clin Microbiol 1993; 31: 2802–2804.
Drumm B, Pérez-Pérez GI, Blaser MJ, Sherman PM. Intrafamilial clustering of Helicobacter pylori infection. N Engl J Med 1990; 322: 359–363.
McCallion WA, Murrary LJ, Bailie AG, et al. Helicobacter pylori infection in children: Relation with current household living conditions. Gut 1996; 39: 18–21.
Webb PM, Knight T, Greaves S, et al. Relations between infection with Helicobacter pylori and living conditions in childhood: Evidence for person to person transmission in early life. Br Med J 1994; 308: 750–753.
Cullen DJE, Collins BJ, Christiansen KJ, et al. When is Helicobacter pylori infection acquired? Gut 1993; 34: 1681–1682.
Banatvala N, Mayo K, Mégraud F, et al. The cohort effect and Helicobacter pylori. J Infect Dis 1993; 168: 219–221.
Dubois A. Spiral bacteria in the human stomach: The gastric Helicobacters. Emerging Infect Dis 1995; 1: 79–85.
Replogle ML, Glaser SL, Hiatt RA, Parsonnet J. Biologic sex as a risk factor for Helicobacter pylori infection in healthy young adults. Am J Epidemiol 1995; 142: 856–863.
Radcliff FJ, Chen M, Lee A. Protective immunization against Helicobacter stimulates long-term immunity. Vaccine 1996; 14: 780–784.
Monath TP, Kleanthous H, Lee CK, et al. Development of recombinant Helicobacter pylori urease as an oral vaccine: current status. Gut 1995; 37(Suppl 1): A52.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Cilla, G., Pérez-Trallero, E., Garcí-Bengoechea, M. et al. Helicobacter pylori infection: A seroepidemiological study in Gipuzkoa, Basque Country, Spain. Eur J Epidemiol 13, 945–949 (1997). https://doi.org/10.1023/A:1007480625665
Issue Date:
DOI: https://doi.org/10.1023/A:1007480625665