Abstract
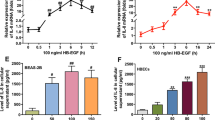
Mitogens of the EGF family may play an important role in regulating the proliferation of airway epithelial cells (AEC). We examined the production of autocrine mitogenic activity by mouse AEC cultured from explants of tracheal tissue. DNA synthesis by growth-arrested AEC was stimulated by conditioned media from cells maintained in serum-free culture without exogenous growth factors. The mitogenic activity was blocked by a specific inhibitor of the EGF receptor tyrosine kinase. Furthermore, conditioned media from AEC contained molecular species that could compete with radiolabeled EGF in a receptor binding assay. However, mitogenic activity was not blocked by neutralizing antibodies to EGF or to transforming growth factor-α, but was partly inhibited by co-incubation with heparin, suggesting that it might be due to a heparin-binding member of the EGF family. The activity was potentiated by co-incubation with IGF-1, analogous to the potentiation by IGF-1 of the mitogenic activity of EGF for AEC. Moreover, the autocrine mitogen produced by AEC exhibited cooperative interaction with the mitogenic activity in conditioned media from growth factor-deprived mouse lung fibroblasts, consistent with the hypothesis that interactions with mesenchymal cells could influence the proliferation of AEC in vivo.
Similar content being viewed by others
References
Barnard JA, Graves-Deal R, Pittelkow MR et al. Auto-and cross-induction within the mammalian epidermal growth factor-related peptide family. J Biol Chem. 1994;269:22817–22.
Besner GE, Whelton D, Crissman-Combs MA, Steffen CL, Kim GY, Brigstock DR. Interaction of heparin-binding EGF-like growth factor (HB-EGF) with the epidermal growth factor receptor: modulation by heparin, heparinase, or synthetic heparin-binding HB-EGF fragments. Growth Factors. 1992;7:289–96.
Cook PW, Mattox PA, Keeble WW et al. A heparin sulfate-regulated human keratinocyte autocrine growth factor is similar or identical to amphiregulin. Mol Cell Biol. 1991; 11:2547–57.
Dlugosz AA, Cheng C, Denning MF, Dempsey PJ, Coffey RJ, Yuspa SH. Keratinocyte growth factor receptor ligands induce transforming growth factor α expression and activate the epidermal growth factor signaling pathway in cultured epidermal keratinocytes. Cell Growth Differ. 1994;5:1283–92.
Erjefalt JS, Erjefalt I, Sundler F, Persson CGA. In vivo restitution of airway epithelium. Cell Tissue Res. 1995;281: 305–16.
Ferriola PC, Robertson AT, Rusnak DW, DiAugustine R, Nettesheim P. Epidermal growth factor dependence and TGFα autocrine growth regulation in primary rat tracheal epithelial cells. J Cell Physiol. 1992;152:302–9.
Jetten AM. Growth and differentiation factors in tracheobronchial epithelium. Am J Physiol. 1991;260:L361–73.
Kumar RK, O'Grady R, Li W, Smith LW, Rhodes GC. Primary culture of adult mouse lung fibroblasts in serum-free medium: response to growth factors. Exp Cell Res. 1991;193:398–404.
Kumar RK, O'Grady R, Di Girolamo N. Epidermal growth factor-like molecular species in normal bronchoalveolar lavage fluid. Lung. 1996;174:171–9.
Kumar RK, Maronesse SE, O'Grady R. Serum-free culture of mouse tracheal epithelial cells. Exp Lung Res. 1997;23:427–40.
Levitzki A, Gazit A. Tyrosine kinase inhibition: an approach to drug development. Science. 1995;267:1782–8.
Miller LA, Cheng LZ, Wu R. Inhibition of epidermal growth factor-like growth factor secretion in tracheobronchial cells by vitamin A. Cancer Res. 1993;53:2527–33.
Rutten MJ, Harmon P, Campbell DR. Insulin enhances epidermal growth factor-and transforming growth factor-α-stimulated growth in primary cultures of guinea pig gastric mucous epithelial cells. Scand J Gastroenterol. 1991;26: 965–73.
Shimizu T, Nishihara M, Kawaguchi S, Sakakura Y. Expression of phenotypic markers during regeneration of rat tracheal epithelium following mechanical injury. Am J Respir Cell Mol Biol. 1994;11:85–94.
Shoji S, Rickard KA, Takizawa H, Ertl RF, Linder J, Rennard SI. Lung fibroblasts produce growth stimulatory activity for bronchial epithelial cells. Am Rev Respir Dis. 1990;141: 433–9.
Stewart AG, Tomlinson PR, Wilson J. Airway wall remodelling in asthma: a novel target for the development of anti-asthma drugs. Trends Pharmacol Sci. 1993;14:275–9.
Tsao MS, Zhu H, Viallet J. Autocrine growth loop of the epidermal growth factor receptor in normal and immortalized human bronchial epithelial cells. Exp Cell Res. 1996; 223:268–73.
Valyi-Nagy I, Jensen PJ, Albelda SM, Rodeck U. Cytokine-induced expression of transforming growth factor-α and the epidermal growth factor receptor in neonatal skin explants. J Invest Dermatol. 1992;99:350–6.
Vardy DA, Kari C, Lazarus GS et al. Induction of autocrine epidermal growth factor receptor ligands in human keratinocytes by insulin/insulin-like growth factor-1. J Cell Physiol. 1995;163:257–65.
Wilhelm DL. Regeneration of tracheal epithelium. J Pathol Bacteriol. 1953;65:543–50.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kumar, R., Maronese, S. & Hassim, Z. Cooperative interaction of autocrine and paracrine mitogens for airway epithelial cells. Cell Biol Toxicol 14, 293–299 (1998). https://doi.org/10.1023/A:1007439109686
Issue Date:
DOI: https://doi.org/10.1023/A:1007439109686