Abstract
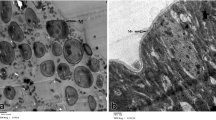
Fungi belonging to the genus Coelomomyces can infect mosquito larvae and develop within the larval hemocoel. To examine fungal development, Aedesaegypti larvae infected with Coelomomyces stegomyiae Keilin were fixed, embedded and sectioned for both light and electron microscopy. While fungal hyphae of C. stegomyiae did not invade cells other than the cuticular epithelial cells, they did penetrate a number of tissues including muscles, midgut, hemopoietic organ, imaginal discs, and Malpighian tubules.
Similar content being viewed by others
References
Lucarotti CJ, Andreadis TG. Reproductive strategies and adaptations for survival among obligatory microsporidian and fungal parasites of mosquitoes: A comparative analysis of Amblyospora and Coelomomyces. J Am Mosq Control Assoc 1995; 11: 111–121.
Whisler HC. Life history of species of Coelomomyces. In Couch JN, Bland CE, eds. The genus Coelomomyces. Academic Press Inc. Orlando, FL, 1985: 9–22.
Travland LB. Initiation of infection of mosquito larvae (Culiseta inornata) by Coelomomyces psorophorae. J Invertebr Pathol 1979b; 33: 95–105.
Zebold SL, Whisler HC, Shemanchuk JA, Travland LB. Host specificity and penetration in the mosquito pathogen Coelomomyces psorophorae. Can J Bot 1979; 57: 2766–2770.
Couch, JN. Sporangium germination of Coelomomyces punctatus and the conditions favoring the infection of Anopheles quadrimaculatus under laboratory conditions. In: Proceeding of the joint U.S.-Japan seminar on control of insect pests. Fukuoka, 1968; 93–105.
Roberts DW. Fungal infections in mosquitoes. In: Aubin A, Belloncik A, Bourassa JP, Lacoursiéve E, Pellisier M, eds. Le contrô le des mosquitoes/mosquito control. Université du Quebec Press, Montreal, PQ, 1974; 143–193.
Lucarotti CJ. Invasion of Aedes aegypti ovaries by Coelomomyces stegomyiae. J Invertebr Pathol 1992; 60: 176–184.
Shoulkamy MA, Lucarotti CJ. Pathology of Coelomomyces stegomyiae in larval Aedes aegypti. Mycologia 1998; 90: 559–564.
Lucarotti CJ, Federici BA. Development and structure of the resting sporangium wall in Coelomomyces dodgei and modification during dehiscence. J Ultrastruct and Mol Struct Res 1985; 95: 96–107.
Madelin MF, Beckett A. The production of planonts by thin walled sporangia of the fungus Coelomomyces indicus. A parasite of mosquitoes. J Gen Microbiol 1972; 72: 185–200.
Martin WW. A morphological and cytological study of development in Coelomomyces punctatus parasitic in Anopheles quadrimaculatus. J Elisha Mitchell Sci Soc 1969; 85: 59–72.
Powell MJ. Ultrastructural changes in the cell surface of Coelomomyces punctatus infecting mosquito larvae. Can J Bot 1976; 54: 1419–1437.
Shoulkamy MA. The occurrence of Coelomomyces stegomyiae in Egypt. Egyp. Proceeding, Sixth Egyptian Botanical Conference, Cairo University, Giza, November 24–26, 1998, Vol. II, 377–382.
Umphlett CJ. Development of the resting sporangia of two species of Coelomomyces. Mycologia 1965; 56: 488–497.
Shoulkamy MA, Lucarotti CJ, El-Ktatny MST, Hassan SKM. Factors affecting Coelomomyces stegomyiae infections in adult Aedes aegypti. Mycologia 1997; 89: 830–836.
Lucarotti CJ, Klein MB. Pathology of Coelomomyces stegomyiae in adult Aedes aegypti ovaries. Can J Bot 1988; 66: 877–884.
Mollenhauer HR. Plastic embedding mixture for use in electron microscopy. Stain Technol 1964; 39: 111–114.
Angerer L, Angerer RC. In situ hyberidization with 35S-labelled RNA probes. DuPont Biotech Update 1989; 4: 2–6.
Trump BF, Smucker EA, Edward A, Benditt EP. A method for staining epoxy sections for light microscopy. J Ultrastruct Res 1961; 5: 343–348.
Lucarotti CJ, Federici BA. Ultrastructure of the gametes of Coelomomyces dodgei Couch (Blastocladiales, Chytridiomycetes). Protoplasma 1984b;121: 77–86.
Shoulkamy MA. Biotic and abiotic factors affecting the development of Coelomomyces stegomyiae in Aedes aegypti larvae and the production of infected adult female mosquitoes. Ph.D. Thesis. Faculty of Science, Minia University, 1996, 106 pp.
Federici BA. Insecticidal bacteria proteins identify the midgut epithelium as a source of novel target sites for insect control. Arch Insect Biochem Physiol 1993; 22: 357–371.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Shoulkamy, M.A., Abdelzaher, H.M. & Shahin, A.A. Ultrastructural changes in the muscles, midgut, hemopoietic organ, imaginal discs and Malpighian tubules of the mosquito Aedes aegypti larvae infected by the fungus Coelomomyces stegomyiae. Mycopathologia 149, 99–106 (2001). https://doi.org/10.1023/A:1007299217926
Issue Date:
DOI: https://doi.org/10.1023/A:1007299217926