Abstract
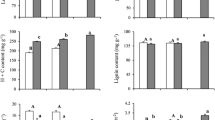
There are significant differences in leaf life-span among evergreen sclerophyllous species and drought semideciduous species growing in the Mediterranean maquis. Cistus incamus, which has a leaf life-span of four-eight months, was characterised by the highest net photosynthetic rates (P N), while Quercus ilex and Phillyrea latifolia, which maintain their leaves two-three and two-four years, respectively, had a lower P N. The longer leaf life-span of the two evergreen sclerophyllous species may be justified to cover the high production costs of leaf protective structures such as cuticle, hairs, and sclereids: cuticle and hairs screen radiation penetrating into the more sensitive tissues, and sclereids have a light-guiding function. Q. ilex and P. latifolia have the highest leaf mass/area ratio (LMA = 209 g m-2) and a mesophyll leaf density (2065 cells per mm2 of leaf cross section area) about two times higher than C. incanus. In the typical evergreen sclerophyllous species the steepest leaf inclination (α = 56°) reduces 42 % of radiation absorption, resulting in a reduced physiological stress at leaf level, particularly in summer. C. incanus, because of its low leaf life-span, requires a lower leaf investment in leaf protective structures. It exhibits a drastic reduction of winter leaves just before summer drought, replacing them with smaller folded leaves. The lower leaf inclination (α = 44°) and the lower LMA (119 g m-2) of C. incanus complement photosynthetic performance. Water use efficiency (WUE) showed the same trend in Q. ilex, P. latifolia, and C. incanus, decreasing 60 % from spring to summer, due to the combined effects of decreased CO2 uptake and increased transpirational water loss.
Similar content being viewed by others
References
Abril, M., Hanano, R.: Ecophysiological responses of three evergreen woody Mediterranean species to water stress.-Acta oecol. 19: 377–387, 1998.
Berg, V.S., Heuchelin, S.: Leaf orientation of soybean seedlings. I. Effect of water potential and photosynthetic photon flux density on paraheliotropism.-Crop Sci. 30: 631–638, 1990.
Björkman, O., Powles, S.B.: Inhibition of photosynthetic reactions under water stress: interaction with light level.-Planta 161: 490–504, 1984.
Bolhàr-Nordenkampf, H.R., Draxler, G.: Functional leaf anatomy.-In: Hall, D.O., Scurlock, J.M.O., Bolhàr-Nordenkampf, H.R., Leegood, R.C., Lang, S.P. (ed.): Photosynthesis and Production in a Changing Environment. A Field and Laboratory Manual. Pp. 91–112. Chapman and Hall, London-Glasgow-New York-Tokyo-Melbourne-Madras 1993.
Catarino, F.M., Correia, O.A., Webb, E., Davis, M.: Morphological and physiological responses of the Mediterranean evergreen sclerophyll, Ceratonia siliqua, to different light intensities.-In: Margaris, N.S., Mooney, H.A. (ed.): Components of Productivity of Mediterranean-Climate Regions. Basic and Applied Aspects. Pp. 5–15. Dr W. Junk Publ., The Hague-Boston-London 1981.
Chabot, B.F., Chabot, J.F.: Effects of light and temperature on leaf anatomy and photosynthesis in Fragaria vesca.-Oecologia 26: 363–377, 1977.
Chabot, B.F., Hicks, D.J.: The ecology of leaf life-spans.-Annu. Rev. Ecol. Syst. 13: 229–259, 1982.
Chapin, F.S., III: The mineral nutrition of wild plants.-Annu. Rev. Ecol. Syst. 11: 233–260, 1980.
Codey, P.D.: Effects of plant growth rate and leaf lifetime on the amount and type of anti-herbivore defense.-Oecologia 74: 531–536, 1988.
Comstock, J.P., Mahall, B.E.: Drought and changes in leaf orientation for two California chaparral shrubs: Ceanothus megacarpus and Ceanothus crassifolium.-Oecologia 65: 531–535, 1985.
Damesin, C., Rambal, S.: Field study of leaf photosynthetic performance by a Mediterranean deciduous oak tree (Quercus pubescens) during a severe summer drought.-New Phytol. 131: 159–167, 1995.
Ehleringer, J.R.: Temperature and energy budgets.-In: Pearcy, R.W., Ehleringer, J., Mooney, H.A., Rundel, P.W. (ed.): Plant Physiological Ecology. Field Methods and Instrumentation. Pp. 117–135. Chapman and Hall, London 1989.
Ehleringer, J.R., Comstock, J.: Leaf absorptance and leaf angle: mechanisms for stress avoidance.-In: Tenhunen, J.D., Catarino, F.M., Lange, O.L., Oechel, W.C. (ed.): Plant Response to Stress. Pp. 547–551. Springer-Verlag, Berlin-Heidelberg-New York-London-Paris-Tokyo 1987.
Ehleringer, J., Mooney, H.A.: Productivity of desert and mediterranean-climate plants.-In: Lange, O.L., Nobel, P.S., Osmond, C.B.L., Ziegler, H. (ed.): Physiological Plant Ecology IV. Pp. 205–231. Springer-Verlag, Berlin-Heidelberg-New York 1983.
Fahn, A.: Plant Anatomy.-Pergamon Press, Oxford 1990.
Gratani, L.: Structural and ecophysiological plasticity of some evergreen species of the mediterranean maquis in response to climate.-Photosynthetica 31: 335–343, 1995.
Gratani, L., Crescente, M.F.: Phenology and leaf adaptative strategies of Mediterranean maquis plants.-Ecol. medit. 23: 11–19, 1997.
Gratani, L., Pesoli, P., Crescente, M.F.: Relationship between photosynthetic activity and chlorophyll content in an isolated Quercus ilex L. tree during the year.-Photosynthetica 35: 445–451, 1998.
Gray, J.T., Schlesinger, W.H.: Nutrient use by evergreen and deciduous shrubs in Southern California. II. Experimental investigations of the relationships between growth, nitrogen uptake and nitrogen availability.-J. Ecol. 71: 43–56, 1983.
Kao, W.-Y., Forseth, I.N.: The effects of nitrogen, light and water availability on tropic leaf movements in soybean (Glycine max).-Plant Cell Environ. 14: 287–293, 1991.
Kao, W.-Y., Forseth, I.N.: Diurnal leaf movement, chlorophyll fluorescence and carbon assimilation in soybean growth under different nitrogen and water availabilities.-Plant Cell Environ. 15: 703–710, 1992.
Karabourniotis, G.: Light-guiding function of foliar sclereids in the evergreen sclerophyll Phillyrea latifolia: a quantitative approach.-J. exp. Bot. 49: 739–746, 1998.
Karabourniotis, G., Kofidis, G., Fasseas, C., Liakoura, V., Drossopoulos, I.: Polyphenol deposition in leaf hairs of Olea europaea (Oleaceae) and Quercus ilex (Fagaceae).-Amer. J. Bot. 85: 1007–1012, 1998.
Karabourniotis, G., Papadopoulos, K., Papamarkou, M., Manetas, Y.: Ultraviolet-B radiation absorbing capacity of leaf hairs.-Physiol. Plant. 86: 414–418, 1992.
Karlsson, P.S.: Photosynthetic capacity and photosynthetic nutrient-use efficiency of Rhododendron lapponicum leaves as related to leaf nutrient status, leaf age and branch reproductive status.-Funct. Ecol. 8: 694–700, 1994.
Kikuzawa, K.: The basic for variation in leaf longevity of plants.-Vegetatio 121: 89–100, 1995.
Koike, T.: Leaf structure and photosynthetic performance as related to the forest succession of deciduous broad-leaved trees.-Plant Species Biol. 3: 77–87, 1988.
Kummerow, J.: Comparative anatomy of sclerophylls of Mediterranean climatic areas.-In: Di Castri, F., Mooney, H.A. (ed.): Mediterranean Type Ecosystems. Origin and Structure. Pp. 157–167. Springer-Verlag, Berlin 1973.
Larcher, W.: Physiological Plant Ecology. 3rd Ed.-Springer-Verlag, Berlin-Heidelberg 1995.
Ludlow, M.M., Björkman, O.: Paraheliotropic leaf movement in Siratro as a protective mechanism against drought-induced damage to primary photosynthetic reactions: damage by excessive light and heat.-Planta 161: 505–518, 1984.
Medina, E., Sobrado, M., Herrera, R.: Significance of leaf orientation for leaf temperature in an Amazonian sclerophyll vegetation.-Radiat. Environ. Biophys. 15: 131–140, 1978.
Miller, P.C., Stoner, W.A.: Canopy structure and environmental interactions.-In: Solbrig, O.T., Jain, S., Johnson, G.B., Raven, P.H. (ed.): Topics in Plant Population Biology. Pp. 428–458. Columbia University Press, New York 1979.
Mitrakos, K., Christodoulakis, N.S.: Leaf structure diversity in Mediterranean evergreen sclerophyllous.-In: Margaris, N.S., Mooney, H.A. (ed.): Components of Productivity of Mediterranean-Climate Regions. Basic and Applied Aspects. Pp. 21–25. Dr W. Junk Publishers, The Hague-Boston-London 1981.
Mooney, H.A., Gulmon, S.L.: Constraints on leaf structure and function in reference to herbivory.-Bioscience 32: 198–206, 1982.
Nobel, P.S.: Form and orientation in relation to PAR interception by cacti and agaves.-In: Givnish, T.J. (ed.): On the Economy of Plant Form and Function. Pp. 83–103. Cambridge University Press, Cambridge-London-New York-New Rochelle-Melbourne-Sydney 1986.
Nobel, P.S., Forseth, I.N., Long, S.P.: Canopy structure and light interception.-In: Hall, D.O., Scurlock, J.M.O., Bolhàr-Nordenkampf, H.R., Leegood, R.C., Long, S.P. (ed.): Photosynthesis and Production in a Changing Environment. A Field and Laboratory Manual. Pp. 79–90. Chapman & Hall, London-Glasgow-New York-Tokyo-Melbourne-Madras 1993.
Oechel, W.C., Lawrence, W., Mustafa, J., Martínez, J.: Energy and carbon acquisition.-In: Miller, P.C. (ed.): Resource Use by Chaparral and Matorral. Pp. 151–183. Springer-Verlag, Berlin-New York-Heidelberg 1981.
Peñuelas, J., Filella, I., Llusia, J., Siscart, D., Piñol, J.: Comparative field study of spring and summer leaf gas exchange and photobiology of the mediterranean trees Quercus ilex and Phillyrea latifolia.-J. exp. Bot. 49: 229–238, 1998.
Powles, S.B., Björkman O.: Leaf movement in the shade species Oxalis oregana. II. Role in protection against injury by intense light.-Carnegie Inst. Washington Year Book 81: 63–66, 1981.
Prichard, J.M., Forseth, I.N.: Rapid leaf movement, microclimate, and water relations of two temperate legumes in three contrasting habitats.-Amer. J. Bot. 75: 1201–1211, 1988.
Reich, P.B., Kloeppel, B.D., Ellsworth, D.S, Walters, M.B.: Different photosynthesis-nitrogen relations in deciduous hardwood and evergreen coniferous tree species.-Oecologia 104: 24–30, 1995.
Reich, P.B., Schoettle, A.W., Stroo, H.F., Troiano, J., Amundson, R.G.: Effects of ozone and acid rain on white pine (Pinus strobus) seedlings grown in five soils. I. Net photosynthesis and growth.-Can. J. Bot. 65: 977–987, 1987.
Reich, P.B., Walters, M.B., Ellsworth, D.S.: Leaf age and season influence the relationships between leaf nitrogen, leaf mass per area, and photosynthesis in maple and oak trees.-Plant Cell Environ. 14: 251–259, 1991.
Reich, P.B., Walters, M.B., Ellsworth, D.S.: Leaf life-span in relation to leaf, plant, and stand characteristics among diverse ecosystems.-Ecol. Monogr. 62: 365–392, 1992.
Skaltsa, H.E., Verycokidou, C., Harvala, C., Karabourniotis, G., Manetas, Y.: UV-B protective potential and flavonoid content of leaf hairs of Quercus ilex.-Phytochemistry 37: 987–999, 1994.
Smith, W.K., Bell, D.T., Shepherd, K.A.: Association between leaf structure, orientation, and sunlight exposure in five Western Australian communities.-Amer. J. Bot. 85: 56–63, 1998.
Tenhunen, J.D., Beyschlag, W., Lange, O.L., Harley, P.C.: Changes during summer drought in leaf CO2 uptake rates of macchia shrubs growing in Portugal: Limitations due to photosynthetic capacity, carboxylation efficiency, and stomatal conductance.-In: Tenhunen, J.D., Catarino, F.M., Lange, O.L., Oechel, W.C. (ed.): Plant Response to Stress. Pp. 305–327. Springer-Verlag, Berlin-Heidelberg-New York-London-Paris-Tokyo 1987.
Turner, I.M.: Sclerophylly: primarily protective?-Funct. Ecol. 8: 669–675, 1994.
Valladares, F., Pearcy, R.W.: Interactions between water stress, sun-shade acclimation, heat tolerance and photoinhibition in the sclerophyll Heteromeles arbutifolia.-Plant Cell Environ. 20: 25–36, 1997.
Van Arendonk, J.J.C.M., Poorter, H.: The chemical composition and anatomical structure of leaves of grass species differing in relative growth rate.-Plant Cell Environ. 17: 963–970, 1994.
Werner, C., Correia, O., Beyschlag, W.: Two different strategies of Mediterranean macchia plants to avoid photoinhibitory damage by excessive radiation levels during summer drought.-Acta oecol. 20: 15–23, 1999.
Williams, K., Field, C.B., Mooney, H.A.: Relationships among leaf construction cost, leaf longevity, and light environment in rain-forest plants of the genus Piper.-Amer. Naturalist 133: 198–211, 1989.
Wuenscher, J.E., Kozlowski, T.T.: Relationship of gas-exchange resistance to tree-seedling ecology.-Ecology 52: 1016–1023, 1971.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gratani, L., Bombelli, A. Leaf Anatomy, Inclination, and Gas Exchange Relationships in Evergreen Sclerophqldous and Drought Semideciduous Shrub Species. Photosynthetica 37, 573–585 (2000). https://doi.org/10.1023/A:1007171525298
Issue Date:
DOI: https://doi.org/10.1023/A:1007171525298