Abstract
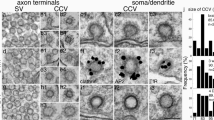
SNAP-25, synaptosomal associated protein of 25 kDa, is reported to be a t-SNARE (target receptor associated with the presynaptic plasma membrane) involved in the docking and fusion of synaptic vesicles. We present here the first ultrastructural localization of SNAP-25 in intact neurons by pre-embedding EM immunocytochemistry in rat brains, hippocampal slice cultures, and PC12 cells. In differentiated neurons, SNAP-25 labeling was clearly membrane-associated. The labeling was most prominent in the plasma membrane of axons and excluded from the plasma membranes of soma and dendrites. Furthermore, SNAP-25 did not appear to be restricted to the synaptic junctions. SNAP-25 labeling was seen in the cytoplasm of the soma and large dendrites, mostly associated with the Golgi complexes. There were also some SNAP-25 labeled tubulo-vesicular structures in the cytoplasm of the soma and the axons, but rarely in the smaller dendrites. In PC12 cells, after 5–10 minutes of high potassium (75 mM) stimulation in the presence of HRP, SNAP-25 labeling appeared, additionally, on HRP-filled early endosomes. After a longer (20–30 minutes) HRP incubation, most of the later stage endosomes and lysosomes were loaded with HRP but they were negative for SNAP-25. These results suggest that SNAP-25 is sorted out of these late endosomal compartments, and that the bulk of the SNAP-25 protein is probably recycled back to the axolemma from the early endosomes. In contrast, in those samples which were incubated with HRP for longer periods, there were still some SNAP-25–positive vesicular structures which were HRP-negative. These structures most likely represent anterograde vesicles that carry newly synthesized SNAP-25 from the soma to the axolemma by axonal transport. SNAP-25 appears to be sorted at the Golgi complex to reach the axolemma specifically. Its widespread distribution all along the axolemma does not support the view of SNAP-25 as a t-SNARE limited for synaptic exocytosis.
Similar content being viewed by others
References
BURGESS, T. L. & KELLY, R. B. (1987) Constitutive and regulated secretion of proteins. Annual Review of Cell Biology 3, 243–294.
CALLI, T., CARCIA, P., MUNDIGL, O., CHILCOTE, T. J. & DE CAMILLI, P. (1995) v-and t-SNAREs in neuronal exocytosis: A need for additional components to define sites for release. Neuropharmacology 34, 1351–1360.
CASTEL, M., MORRIS, J. & BELENKY, M. (1996) Nonsynaptic and dendritic exocytosis from dense-cored vesicles in the suprachiasmatic nucleus. NeuroReport 7, 543–547.
DUC, C. & CATSICAS, S. (1995) Ultrastructural localization of SNAP-25 within the rat spinal cord and peripheral nervous system. Journal of Comparative Neurology 356, 152–163.
FUTTER, C. E., GIBSON, A., ALLCHIN, E. H., MAXWELL, S., RUDDOCK, L. J., ODORIZZI, G., DOMINGO, D., TROWBRIDGE, I. S. & HOPKINS, C. R. (1998) In polarized MDCK cells basolateral vesicles arise from clathrin-gama-adaptin-coated domains on endosomal tubules. Journal of Cell Biology 141, 611–623.
GARCIA, E. P., MCPHERSON, P. S., CHILOCOTE, T. J., TAKEI, K. & DE CAMILLI, P. (1995) rbSec1A and B colocalize with synataxin 1 and SNAP-25 throughout the axon, but are not in a stable complex with syntaxin. Journal of Cell Biology 129, 105–120.
LI, J.-Y., JAHN, R., DAHLSTRÖM, A. (1996) Axonal transport and targeting of the t-SNAREs SNAP-25 and syntaxin 1 in the peripheral nervous system. European Journal of Cell Biology 70, 12–22.
LOEWY, A., LIU, W. S., BAITINGER, C. & WILLARD, M. B. (1991) The major 35S-methionine-labeled rapidly transported protein (Superprotein) is identical to SNAP-25, a protein of synaptic terminals. Journal of Neuroscience 11, 3412–3421.
LYSAKOWSKI, A., FIGUERAS, H., PRICE, S. D. & PENG, Y. Y. (1999) Dense-cored vesicles, smooth endoplasmic reticulum, and mitochondria are closely associated with non-specialized parts of plasma membrane of nerve terminals: Implications for exocytosis and calcium buffering by intraterminal organelles. Journal of Comparative Neurology 403, 378–390.
MCMAHON, H. T., SUDHOF, T. C. (1995) Synaptic core complex of synaptobrevin, synatxin, and SNAP-25 forms high affinity alpha-SNAP binding site. Journal of Biological Chemistry 270, 2213–2217.
NIRENBERG, M. J., VAUGHAN, R. A., UHL, G. R., KUHAR, M. J. & PICKEL, V. M. (1996) The dopamine transporter is localized to dendrite and axonal plasma membranes of nigrostriatal dopaminergic neurons. Journal of Neuroscience 16, 436–447.
NUSSER, Z., SIEGHART, W., STEPHENSON, F. A. & SOMOGYI, P. (1996) The alpha-6 subunit of the GABA A receptor is concentrated in both inhibitory and excitatory synapses on cerebellar granule cells. Journal of Neuroscience 16, 103–114.
OYLER, G. A., HIGGINS, G. A., HART, R. A., BATTENBERG, E., BILLINGSLEY, M., BLOOM, F. E., WILSON, M. C. (1989) The identification of a novel synaptosomal-associated protein, SNAP-25, differentially expressed by neuronal subpopulations. Journal of Cell Biology 109, 3039–3052.
SIMONS, K. & IKONEN, E. (1997) Sphingolipid-cholesterol rafts in membrane trafficking and signaling. Nature 387, 569–572.
SKENE, J. H. P. (1989) Axonal growth-associated proteins. Annual Review of Neuroscience 12, 127–156.
SOLLNER, T., WHITEHEART, S. W., BRUNNER, M., ERDJUMENT-BROMAGE, H., GEROMANOS, S., TEMPST, P. & ROTHMAN, J. E. (1993) SNAP receptors implicated in vesicle targeting and fusion. Nature 362, 318–324.
STOPPINI, L., BUSHS, P. A. & MULLER, D. (1991) A simple method for organotypic cultures of nervous tisssue. Journal of Neuroscience Methods 37, 173–182.
TAO-CHENG, J.-H., DOSEMECI, A., BRESSLER, J. P., BRIGHTMAN, M. W. & SIMPSON, D. L. (1995) Characterization of synaptic vesicles and related neuronal features in NGF and ras oncogene differentiated PC12 cells. Journal of Neuroscience Research 42, 323–334.
TAO-CHENG, J.-H. & EIDEN, L. E. (1997) The vesicular monoamine transporter VMAT2 and vesicular acetylcoholine transporter VAChT are sorted to separate vesicle populations in PC12 cells. In Advances in Pharmacology Vol. 42, (edited by GOLDSTEIN, D., EISENHOFER, G. AND MCCARTY, R.), pp. 250–253. Academic Press.
TAO-CHENG, J.-H. & ZHOU, F. C. (1999) Differential polarization of serotonin transporters in axons versus somadendrites: An immunogold electron microscopy study. Neuroscience 94, 821–830.
TANNER, V., PLOUG, T. & TAO-CHENG, J.-H. (1996) Subcellular localization of SV2 and other secretory vesicle components in PC12 cells by an efficient method of pre-embedding EM irmunocytochemistry for cell cultures. Journal of Histochemistry and Cytochemistry 144, 1481–1488.
TOOZE, J. & HOLLINSHEAD, M. (1991) Tubular early endosomeal networks inAT20 and other cells. Journal of Cell Biology 115, 635–653.
WALCH-SOLIMENA, C., BLASI, J., EDELMANN, L., CHAPMAN, E. R., VON MOLLARD, G. F. & JAHN, R. (1995) The t-SNAREs syntaxin 1 and SNAP-25 are present on organelles that participate in synaptic vesicle recycling. Journal of Cell Biology 128, 637–645.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tao-Cheng, JH., Du, J. & McBain, C.J. Snap-25 is polarized to axons and abundant along the axolemma: an immunogold study of intact neurons. J Neurocytol 29, 67–77 (2000). https://doi.org/10.1023/A:1007168231323
Issue Date:
DOI: https://doi.org/10.1023/A:1007168231323