Abstract
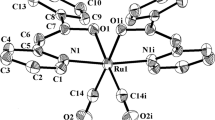
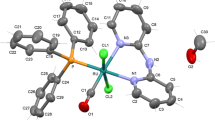
The compound [RuCl2(CO)(DMA)(PPh3)2] [DMA = dimethylacetamide] was obtained from [RuCl3(PPh3)2-(DMA)] · DMA and CO in DMA. Orange crystals of [RuCl2(CO)(DMA)(PPh3)2] · 1/2CH2Cl2 were isolated by slow evaporation of a CH2Cl2/DMA solution and its structure was determined by single crystal X-ray diffraction. The analogous compounds containing DMF and DMSO were obtained from the precursor ttt-[RuCl2(CO)2(PPh3)2]. Characterization of the other complexes is based on i.r. and n.m.r. spectroscopy, including 31P{1H} data.
Similar content being viewed by others
References
E.A. Seddon and K.R. Seddon, The Chemistry of Ruthenium, Elsevier, Amsterdam, 1984.
B.R. James, L.K. Thompson and D.K.W. Wang, Inorg. Chim. Acta, 29, L237 (1978); T.W. Dekleva, I.S. Thorburn and B.R. James, Inorg. Chim. Acta, 100, 49 (1985).
A.A. Batista, O.M. Porcu, O.R. Nascimento, V.M. Barbosa and G. Oliva, J. Coord. Chem., 30, 345 (1993); I.S. Thorburn, S.J. Rettig and B.R. James, Inorg. Chem., 25, 234 (1986).
B.R. James, L.D. Markham, B.C. Hui and G.L. Rempel, J. Chem. Soc., Dalton Trans., 2247 (1973).
Enraf-Nonius. CAD-4-PC. Version 1.2, Enraf-Nonius: Delft, The Netherlands, 1993.
K. Harms and S. Wocadlo. XCAD-4. Program for Processing CAD-4 Diffractometer Data. University of Marburg: Marburg, Germany, 1995.
G.M. Sheldrick. SHELXS-97. Program for Crystal Structure Resolution. Univ. Göttingen: Göttingen, Germany, 1997.
G.M. Sheldrick. SHELXL-97. Program for Crystal Structures Analysis. Univ. Göttingen: Göttingen, Germany, 1997.
L.J. Farrugia. ORTEP3 for Windows. J. Appl. Crystallogr., 30, 565 (1997).
D.T. Cromer and J.B. Mann, Acta Crystallogr., A24, 321 (1968).
T.A. Stephenson and G. Wilkinson, J. Inorg. Nucl. Chem., 28, 945 (1966).
D.A. Perrin, W.L.F. Amarrego and D.F. Perrin, Purification of Laboratory Chemicals, 1st edit., New York, Pergamon Press, 1966.
K. Wohnrath, A.A. Batista, A.G. Ferreira, J. Zukerman-Schpector, L.A.A. De Oliveira and E.E. Castellano, Polyhedron, 17, 2013 (1998).
L.M. Wilkes, J.H. Nelson, J.P. Mitchener, M.W. Babich, W.C. Riley, B.J. Helland, R.A. Jacobson, M.Y. Cheng, K. Seff and L.B. McCusker, Inorg. Chem., 21, 1376 (1982).
F.A. Coton and G. Wilkinson, Advanced Inorganic Chemistry, 5th edit., Wiley-Interscience, Toronto, 1988, p. 1299.
K. Nakamoto, Infrared and Raman Spectra of Inorganic and Coordination Compounds, 5th edit., England, 1997.
M.G. Bhowon, H.L.K. Wah, R. Narain, Polyhedron, 18, 341 (1998); M.B. Egorova, A.V. Drobachenko and A.M. Popov, Koordinatsionnaya Khimiya, 13, 541 (1987); N.P.G. Roeges, A Guide to the Interpretation of Infrared Spectra of Organic Structures. Wiley, England, 1994.
P. Ford, D.F. Rudd, R. Gaunder and H. Taube, J. Am. Chem. Soc., 90, 1187 (1968).
A.M. Zwickel and C. Creuz, Inorg. Chem., 10, 2395 (1971).
J. Chakravarty and S. Bhattacharya, Polyhedron, 13, 2671 (1994).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Batista, A.A., Wonrath, K., Queiroz, S.L. et al. New routes to carbonyl complexes of general formula [RuCl2(CO)(S)(PPh3)2] (S = DMA, DMF, DMSO): crystal structure of [RuCl2(CO)(DMA)(PPh3)2] · 1/2CH2Cl2. Transition Metal Chemistry 26, 365–368 (2001). https://doi.org/10.1023/A:1007129529341
Issue Date:
DOI: https://doi.org/10.1023/A:1007129529341