Abstract
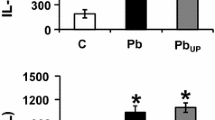
Secretion of Monocyte Chemotactic Protein-1 (MCP-1) by fibroblasts infected with Toxoplasma gondii was studied in vitro. A significantly higher MCP-1 secretion was observed 24 h after infection by live tachyzoites. Analysis of chemokine mRNA transcripts by RNase protection assay revealed that this MCP-1 secretion seems associated with increased MCP-1 mRNA expression. However, these increased levels of MCP-1 secretion and expression were not obtained after stimulation by heat-killed tachyzoites or parasites pre-treated by a specific inhibitor of phosphatidylcholine-specific phospholipase C (D609). Inhibition of parasite multiplication by pyrimethamine did not modify MCP-1 secretion. Thus, it appeared that the active penetration of T. gondii in cells was of major importance in the induction of MCP-1 secretion. None of the other chemokines studied by RNase protection assay (lymphotactin, RANTES, IP-10, MIP-1α, MIP-1β, IL-8, and I-309) were expressed after infection by live tachyzoites. We also found that MCP-1 secretion induced by live T. gondii is blocked by inhibitors of nuclear factor (NF)-κ activation, ALLN and MG132. Such data indicate that NF-κB could be involved in T. gondii-induced MCP-1 production. MCP-1 secretion may contribute to the recruitment of monocytes and lymphocytes and thus participate in the control of T. gondii infection and in its pathogenesis.
Similar content being viewed by others
References
Amichay D, Gazzinelli RT, Karupiah G, Moench TR, Sher A, Farber JM: Genes of chemokines MuMig and Crg-2 are induced in protozoan and viral infections in response to IFN-γ with patterns of tissue expression that suggest non redundant roles in vivo. J Immunol 157: 4511-4520, 1996
Gazzinelli RT, Amichay D, Sharton-Kersten T, Grunwald T, Farber JM, Sher A: Role of macrophage-derived cytokines in the induction and regulation of cell-mediated immunity to Toxoplasma gondii. In: U. Gross (ed). Toxoplasma gondii. Curr Top Microbiol Immunol, Würzburg, Germany, 1996, pp 127-139
Gazzinelli RT, Talvani A, Camargo MM, Santiago HC, Oliveira MAP, Vieira LQ, Martins GA, Aliberti JCS, Silva JS: Induction of cell-mediated immunity during early stages of infection with intracellular protozoa. Braz J Med Biol Res 31: 89-104, 1998
Mannheimer SB, Hariprashad J, Stoeckle MY, Murray HW: Induction of macrophage antiprotozoal activity by monocyte chemotactic and activating factor. FEMS Immunol Med Microbiol 14: 59-61, 1996
Denney CF, Eckmann L, Reed SL: Chemokines secretion of human cells in response to Toxoplasma gondii infection. Infect Immun 67: 1547-1552, 1999
Bliss SK, Marshall AJ, Zhang Y, Denkers EY: Human polymorphonuclear leukocytes produce IL-12, TNF-α, and the chemokines macrophage-inflammatory protein-1α and-1β in response to Toxoplasma gondii antigens. J Immunol 162: 7369-7375, 1999
Rollins BJ: Chemokines. Blood. 90: 909-938, 1997
Michelson S, Dal Monte P, Zipeto D, Bodaghi B, Laurent L, Oberlin E, Arenzana-Seisdedos FA, Virelizier J L, Landini MP: Modulation of RANTES production by human cytomegalovirus infection of fibroblasts. J Virol 171: 6495-6500, 1997
Jiang Y, Russell T R, Graves DT, Cheng H, Nong SH, Levtz SM: Monocyte chemoattractant protein-1 and Interleukin-8 production in mononuclear cells stimulated by oral microorganisms. Infect Immun 64: 4450-4455, 1996
Schluger NW, Rom WN: Early responses to infection: chemokines as mediators of inflammation. Curr Opin Immunol 9: 504-508, 1997
Batolado R, Sacks DL, Savoia D, Musso T: Leishmania major: Infection of human monocytes induces expression of IL-8 and MCAF. Exp Parasitol 82: 21-26, 1996
Burgmann H., Hollenstein U, Thalhammer F, Looareesuwan S, Graninger, W: Serum concentration of MIP-1α and Interleukin-8 in patients suffering from acute Plasmodium falciparum malaria. Clin Immunol Immunopathol 76: 32-36, 1995
Chensue SW, Warmington KS, Lukacs NW, Lincoln NPM, Burdick MD, Strieter RM, Kunkel SL: Monocyte chemotactic protein expression during schistosome egg granuloma formation. Am J Pathol 146: 130-138, 1995
Chensue SW, Warmington KS, Ruth JH, Sanghi PS, Lincoln P, Kunkel L: Role of monocyte chemoattractant protein-1(MCP-1) in Th1 (mycobacterial) and Th2 (schistosomal) antigeninduced granuloma formation. J Immunol 157: 4602-4608, 1996
Racoosin EL, Beverley SM: Leishmania major: Promastigotes induce expression of a subset of chemokine gene in murine macrophages. Exp Parasitol 85: 283-295, 1997
Reale M, Frydas S, Barbacane RC, Placido FC, Cataldo I, Vacalis D, Trakatellis A, Anogianakis G, Felaco M, Di Gioacchino M, Conti P: Induction of monocyte chemotactic protein-1 (MCP-1) and TNF alpha by Trichinella spiralis in serum of mice in vivo. Moll Cell Biochem 179: 1-5, 1998
Rollins BJ, Baggiolini M: Recombinant human MCP-1/JE induces chemotaxis, calcium flux, and the respiratory burst in human monocytes. Blood 78: 1112-1116, 1991
Ricard J, Pelloux H, Favier AL, Gross U, Brambilla E, Ambroise-Thomas P: Toxoplasma gondii: Role of the phosphatidylcholinespecific phospholipase C during cell invasion and intracellular development. Exp Parasitol 91: 231-233, 1999
Ricard J, Pelloux H, Pathak S, Pipy B, Ambroise-Thomas P: TNF-α enhances Toxoplasma gondii cyst formation in human fibroblasts through the sphingomyelinase pathway. Cell Signal 8: 439-432, 1996
Haque S, Haque A, Kasper LH: A Toxoplasma gondii-derived factor(s) stimulates immune downregulation: An in vitro model. Infect Immun 63: 3442-3447, 1995
Amtmann E: The antiviral, antitumoral xanthate D609 is a competitive inhibitor of phosphatidylcholine-specific phopholipase C. Drugs Exptl Clin Res 22: 287-294, 1993
Larsen CG, Zachariae COC, Oppenheim JJ, Matsushima K: Production of monocyte chemotactic and activating factor (MCAF) by human dermal fibroblasts in response to Interleukin-1 or tumor necrosis factor. Biochem Biophys Res Commun 160: 1403-1408, 1989
Yoshimura T, Leonard EJ: Secretion by human fibroblasts of monocyte chemoattractant protein-1, the product of gene JE. J Immunol 144: 2377-2383, 1990
LI ZY, Manthey CL, Perera PH, Sher A, Vogel SN: Toxoplasma gondii soluble antigen induces a subset of lipopolysaccharideinducible genes and tyrosine phosphoproteins in peritoneal macrophages. Infect Immun 32: 3434-3440, 1994
Araujo FG, Huskinson J, Remington JS: Remarkable in vitro and in vivo activities of the hydroxynaphthoquinone 566C80 against tachyzoites and tissue cysts of Toxoplasma gondii. Antimicrob Agents Chemother 35: 293-299, 1991
Romand S, Bryskier A, Moutot M, Derouin F: In vitro and in vivo activities of roxithromycin in combination with pyrimethamine or sulphadiazine against Toxoplasma gondii. J Antimicrob Chemoth 35: 821-832, 1995
Pelloux H, Pernod G, Polack B, Coursange E, Ricard J, Verna JM, Ambroise-Thomas P: Influence of cytokines of Toxoplasma growth in human astrocytoma-derived cells. Parasitol Res 82: 598-603, 1996
Lane TE, Fox HS, Buchmeier MJ: Inhibition of nitric oxide synthase-2 reduces the severity of mouse hepatitis virus-induced demyelinisation: implication for NOS2/No regulation of chemokine expression and inflammation. J Neurovirol 5: 48-54, 1999
Ueda A, Okuda K, Ohno S, Shirai A, Igarashi T, Matsunaga K, Fukushima J, Kawamoto S, Ishigatsubo Y, Okubo T: NF-κB and Sp1 regulate transcription of the human monocyte chemoattractant protein-1 gene. J Immunol 153: 2052-2063, 1994
Parry CN, Martin T. Felts KA, Cobb RR: IL-1β-induced monocyte chemoattractant protein-1 gene expression in endothelial cells is blocked by proteasome inhibitors. Arterioscler Thromb Vasc Biol 18: 934-940, 1999
Ebdnet K, Brown KD, Siebenlist UK, Simon MM, Shaw S: Borrelia burgdorferi activated nuclear factor-kappa B and is a potent inducer of chemokine and adhesion molecule gene expression in endothelial cells and fibroblasts. J Immunol 168: 3285-3292, 1997
Thomas LH, Friedland JS, Sharland M, Becker S: Respiratory syncytial virus-induced RANTES production from human bronchial epithelial cells is dependent on nuclear factor-kappa B nuclear binding and is inhibited by adenovirus-mediated expression of inhibitor of kappa B alpha. J Immunol 161: 1007-1016, 1998
Beauparlant P, Hiscott J: Biological and biochemical inhibitors of the NF-κB/Rel proteins and cytokine synthesis. Cyt Grow Fact Rev 7: 175-190, 1996
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Brenier-Pinchart, MP., Pelloux, H., Simon, J. et al. Toxoplasma gondii induces the secretion of monocyte chemotactic protein-1 in human fibroblasts, in vitro. Mol Cell Biochem 209, 79–87 (2000). https://doi.org/10.1023/A:1007075701551
Issue Date:
DOI: https://doi.org/10.1023/A:1007075701551