Abstract
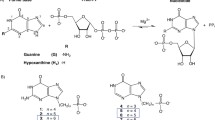
Since the α-D-galactose-(1→3)-D-galactose epitope has been identified to be the major target in the process of hyperacute rejection of xenografts transplanted from nonprimate donors to humans, specific inhibitors of α-galactosyltransferases are of broad interest. Using Trypanosoma brucei, a protozoan parasite causing sleeping sickness and Nagana, we have a very useful model system for the investigation of α-galactosyltransferase inhibitors, since the variant surface glycoprotein (VSG) accounts for about 10% of the total cell protein an this parasite expresses many different galactosyltransferases including the one catalysing the formation of the Galα1→3Gal epitope. In order to study inhibition of galactosylation on the VSG from Trypanosoma brucei, we designed, synthesized and tested substrate analogues of trypanosomal α-galactosyltransferases. Effective inhibitors were a pair of diastereoisomeric UDP-galactose analogs, in which the galactose residue is linked to UDP via a methylene bridge rather than an ester linkage. Hence, galactose cannot be transferred to the respective acceptor substrate VSG or the synthetic acceptor substrate Manα1→6Manα1S-(CH2)7-CH3, which was previously proven to replace VSG effectively [Smith et al. (1996) J Biol Chem 271:6476–82]. Inhibitors have been prepared starting from 1-formyl galactal. The final condensation was performed using UMP morpholidate leading to a pair of diastereomeric compounds in 39% or 30% yield, respectively. These compounds were tested using α-galactosyltransferases prepared from T. brucei membranes and lactose synthetase from bovine milk. While the KM-value for UDP-galactose was determined as 59 µM on bovine lactose synthetase, the KI-values for both inhibitors were 0.3 mM and 1.1 mM respectively, showing that these inhibitors are unable to inhibit enzyme activity significantly. However, using the N-glycan specific α-galactosyltransferase from trypanosomes, the KM-value was determined as 20 µM, while the KI-values were 34 µM and 21 µM respectively. Interestingly, other trypanosomal α-galactosyltransferases, which modify the GPI membrane anchor, are 2 orders of magnitude less effected by the inhibitor.
Similar content being viewed by others
References
van Denderen BJ, Salvaris E, Romanella M, Aminian M, Katerelos M, Tange MJ, Pearse MJ, d'Apice AJ (1979) Transplantation 64: 882–88.
Osman N, McKenzie IFC, Ostenried K, Ionnou YA, Desnick RJ, Sandrin MS (1997) Proc Natl Acad Sci (USA) 94: 14677–82.
Ohdan H, Yang YG, Sykes M (1999) Transplant Proc 31: 945–46.
Vanhove B, Charreau B, Cassard A, Pourcel C, Soulillou JP (1998) Transplantation 66: 1477–85.
Pearse MJ, Witort E, Mottram P, Han W, Murray-Segal L, Romanella M, Salvaris E, Shinkel TA, Goodman DJ, d'Apice AJ (1998) Transplantation 66: 748–54.
Bracy JL, Sachs DH, Iacomini J (1998) Science 281: 1845–47.
Sandrin MS, Vaughan HA, Dabkowski PL, McKenzie LF (1993) Proc Natl Acad Sci (USA) 90: 11391–95.
Galili U, Shohet SB,Kobrin E, Stults CL, Macher BA(1988) J Biol Chem 263: 17755–62.
Vaughan HA, Loveland BE, Sandrin MS (1994) Transplantation 58: 879–82.
Galili U, Macher BA, Buehler J, Shohet SB (1985) J Exp Med 162: 573–82.
Good AH, Cooper DK, Malcolm AJ, Ippolito RM, Koren E, Neethling FA, Ye Y, Zuhdi N, Lamontagne LR (1992) Transplant Proc 24: 559–62.
Dalmasso AP, Vercellotti GM, Fischel RJ, Bolman RM, Bach FH, Platt JL (1992) Am J Pathol 140: 1157–66.
Ferguson MAJ, Homans SW, Dwek RA, Rademacher TW (1988) Science 239: 753–59.
Pingel S, Duszenko M (1992) Biochem J 283: 479–85.
Pingel S, Field RA, Güther MLS, Duszenko M, Ferguson MAJ (1995) Biochem J 309: 877–82.
Cross, GAM (1996) BioEssays 18: 283–91.
Pingel S, Rheinweiler U, Kolb V, Duszenko M (1998) Biochem J 338: 545–51.
Smith TK, Cottaz S, Brimacombe JS, Ferguson MAJ (1996) J Biol Chem 271: 6476–82.
Ziegler T, Dettmann R, Duszenko M, Kolb V (1996) Carbohydr Res 295: 7–23.
Frische K, Schmidt RR (1994) Liebigs Ann Chem 297–303.
Dettinger H, Kurz G, Lehmann J (1979) Carbohydr Res 74: 301–07.
Meier C, Laux WHG, Bats JW, (1995) Liebigs Ann Chem 1963–1979; and references therein
Dale JA, Mosher HS (1973) J Am Chem Soc 95: 512–19.
Hammerschmidt F, Li YF (1994) Tetrahedron 50: 10253–64; and references therein.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kolb, V., Amann, F., Schmidt, R.R. et al. Specific inhibition of an α-galactosyltransferase from Trypanosoma brucei by synthetic substrate analogues. Glycoconj J 16, 537–544 (1999). https://doi.org/10.1023/A:1007026122209
Issue Date:
DOI: https://doi.org/10.1023/A:1007026122209
- Trypanosoma brucei
- α-galactosyltransferases
- sugar donor analogs
- competitive inhibitors
- UDP, uridine-5′-diphosphate
- UDP-Gal, uridine-5′-diphosphate galactose
- VSG, variant surface glycoprotein
- αGalT, α-galactosyltransferase
- Galα1,3GalT, UDP-Gal:β-d-Gal(1 → 4)-D-GlcNAc-α(1,3)-galactosyltransferase (EC 2.4.1.51)
- Galα1,3ManT, UDP-Gal:GPI-anchor-α(1,3)-galactosyltransferase
- Man, D-mannose
- Gal, D-galactose
- Man2-S-C8, Manα(1-6)Manα1S-(CH2)7-CH3
- GlcNAc, D-N-acetylglucosamine
- LacNAc, D-N-acetyllactosamine
- Galα1,3Gal, α-d-Gal(1→3)-D-Gal epitope
- GPI, glycosyl-phosphatidylinositol
- DTT, dithiothreitol
- PIPES, piperazine-N,N′-bis(2-ethanesulfonic acid)