Abstract
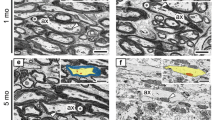
The jimpy mutation of the X-linked proteolipid protein (Plp) gene causes dysmyelination and premature death of the mice. The established phenotype is characterised by severe hypomyelination, increased numbers of dead oligodendrocytes and astrocytosis. The purpose of this study was to define the earliest cellular abnormalities in the cervical spinal cord. We find that on the first and third postnatal days the amount of myelin in jimpy spinal cord is approximately 20% of wild-type. However, the total glial cell density, the number of dead glial cells and the number and distribution of Plp-positive cells, as assessed by in situ hybridization, are similar to wild-type during the first week of life. Immunostaining of cryosections has identified that jimpy spinal cords express on schedule, a variety of antigens associated with mature oligodendrocytes. Dissociated oligodendrocytes, cultured for 18 hours to reflect their in vivo differentiation, express MBP and surface myelin-associated glycoprotein at the same frequency as wild-type. By comparison, the proportion of jimpy oligodendrocytes expressing surface myelin/oligodendrocyte glycoprotein is reduced by approximately 34%. In vivo, however, only a small minority of axons is surrounded by a collar of myelin-associated glycoprotein, suggesting that the majority of jimpy oligodendrocytes fail to make appropriate ensheathment of axons. Although the DM20 isoform is expressed in the embryonic CNS prior to myelin formation, the cellular abnormalities appear to correspond to the time at which the Plp isoform becomes predominant. The results suggest that the primary abnormality in jimpy is the inability of oligodendrocytes to properly associate with, and then ensheath, axons and that oligodendrocyte death compounds, rather than initiates, the established phenotype.
Similar content being viewed by others
References
Anderson, T. J., Schneider, A., Barrie, J. A., Klugmann, M., Mcculloch, M. C., Kirkham, D., Kyriakides, E., Nave, K.-A. & Griffiths, I. R. (1998) Late-onset neurodegeneration in mice with increased dosage of the proteolipid protein gene. Journal of Comparative Neurology 394, 506–519.
Barres, B. A. & Raff, M. C. (1994) Control of oligodendrocyte number in the developing rat optic nerve. Neuron 12, 935–942.
Barres, B. A., Hart, I. K., Coles, H. S. R., Burne, J. F., Voyvodic, J. T., Richardson, W. D. & Raff, M. C. (1992a) Cell death and control of cell survival in the oligodendrocyte lineage. Cell 70, 31–46.
Barres, B. A., Hart, I. K., Coles, H. S. R., Burne, J. F., Voyvodic, J. T., Richardson, W. D. & Raff, M. C. (1992b) Cell death in the oligodendrocyte lineage. Journal of Neurobiology 23, 1221–1230.
Barres, B. A., Jacobson, M. D., Schmid, R., Sendtner, M. & Raff, M. C. (1993a) Does oligodendrocyte survival depend on axons. Current Biology 3, 489–497.
Barres, B. A., Schmid, R., Raff, M. C. & Sendtner, M. (1993b) Multiple extracellular signals are required for long-term oligodendrocyte survival. Development 118, 283–295.
Delassalle, A., Zalc, B., Lachapelle, F., Raoul, M., Collier, P. & Jacque, C. (1981) Regional distribution of myelin basic protein in the central nervous system of quaking, jimpy and normal mice during development and ageing. Journal of Neuroscience Research 6, 303–313.
Dentinger, M. P., Barron, K. D. & Csiza, C. K. (1982) Ultrastructure of the central nervous system in a myelin deficient rat. Journal of Neurocytology 11, 671–691.
Dickinson, P. J., Fanarraga, M. L., Griffiths, I. R., Barrie, J. A., Kyriakides, E. & Montague, P. (1996) Oligodendrocyte progenitors in the embryonic spinal cord express DM-20. Neuropathology and Applied Neurobiology 22, 188–198.
Duncan, I. D., Hammang, J. P., Goda, S. & Quarles, R. H. (1989) Myelination in the jimpy mouse in the absence of proteolipid protein. Glia 2, 148–154.
Duncan, I. D., Paino, C., Archer, D. R. & Wood, P. M. (1992) Functional capacities of transplanted cellsorted adult oligodendrocytes. Developmental Neuroscience 14, 114–122.
Fanarraga, M. L., Griffiths, I. R., Mcculloch, M. C., Barrie, J. A., Cattanach, B. M., brophy, P. J. & Kennedy, P. G. E. (1991) Rumpshaker: an X-linked mutation affecting CNS myelination. A study of the female heterozygote. Neuropathology and Applied Neurobiology 17, 289–297.
Fanarraga, M. L., Sommer, I., Griffiths, I. R., Montague, P., Groome, N. P., Nave, K.-A., Schneider, A., Brophy, P. J. & Kennedy, P. G. E. (1993) Oligodendrocyte development and differentiation in the rumpshaker mutation. Glia 9, 146–156.
Gardinier, M. V., Macklin, W. B., Diniak, A. J. & Deininger, P. L. (1986) Characterization of myelin proteolipid mRNAs in normal and jimpy mice. Molecular and Cellular Biology 6, 3755–3762.
Ghandour, M. S. & Skoff, R. P. (1988) Expression of galactocerebroside in developing normal and jimpy oligodendrocytes in situ. Journal of Neurocytology 17, 485–498.
Gow, A., Friedrich, V. L., JR. & Lazzarini, R. A. (1994a) Intracellular transport and sorting of the oligodendrocyte transmembrane proteolipid protein. Journal of Neuroscience Research 37, 563–573.
Gow, A., Friedrich, V. L., JR. & Lazzarini, R. A. (1994b) Many naturally occurring mutations of myelin proteolipid protein impair its intracellular transport. Journal of Neuroscience Research 37, 574–583.
Gow, A. & Lazzarini, R. A. (1996) Acellular mechanism governing the severity of Pelizaeus-Merzbacher disease. Nature Genetics 13, 422–428.
Gow, A., Southwood, C. M. & Lazzarini, R. A. (1998) Disrupted proteolipid protein trafficking results in oligodendrocyte apoptosis in an animal model of Pelizaeus-Merzbacher disease. Journal of Cell Biology 140, 925–934.
Griffiths, I. R., Duncan, I. D. & Mcculloch, M. (1981) Shaking pup: a disorder of central myelination in the spaniel dog. II. Ultrastructural observations on the white matter of cervical spinal cord. Journal of Neurocytology 10, 847–858.
Griffiths, I. R., Klugmann, M., Anderson, T. J., Yool, D., Thomson, C. E., Schwab, M. H., Schneider, A., Zimmermann, F., Mcculloch, M. C., Nadon, N. L. & Nave, K.-A. (1998) Axonal swellings and degeneration in mice lacking the major proteolipid of myelin. Science 280, 1610–1613.
Griffiths, I. R., Kyriakides, E. & Abrahams, S. (1989a) The distribution of MAG in association with the axonal lesions of canine progressive axonopathy. Journal of Neurocytology 18, 353–358.
Griffiths, I. R., Mitchell, L. S., Mcphilemy, K., Morrison, S., Kyriakides, E. & Barrie, J. A. (1989b) Expression of myelin protein genes in Schwann cells. Journal of Neurocytology 18, 345–352.
Griffiths, I. R., Scott, I., Mcculloch, M. C., Barrie, J. A., Mcphilemy, K. & Cattanach, B. M. (1990) Rumpshaker mouse: a new X-linked mutation affecting myelination: evidence for a defect in PLP expression. Journal of Neurocytology 19, 273–283.
Hodes, M. E., Pratt, V. M. & Dlouhy, S. R. (1993) Genetics of Pelizaeus-Merzbacher disease. Developmental Neuroscience 15, 383–394.
Kagawa, T., Nakao, J., Yamada, M., Shimizu, K., Hayakawa, T., Mikoshiba, K. & Ikenaka, K. (1994) Fate of jimpy-type oligodendrocytes in jimpy heterozygote. Journal of Neurochemistry 62, 1887–1893.
Kagawa, T., Oba, A., Okumura, S. & Ikenaka, K. (1996) Localization of mRNA for UDP-galactose: Ceramide galactosyltransferase in the brain during mouse development. Developmental Neuroscience 18, 309–318.
Kalwy, S. A., Smith, R. & Kidd, G. J. (1997) Myelin proteolipid protein expressed in COS-1 cells is targeted to actin-associated surfaces. Journal of Neuroscience Research 48, 201–211.
King, H., Mcculloch, M. C., Barrie, J. A., Kyriakides, E., Cattanach, B. M., Beechey, C. V. & Griffiths, I. R. (1997) Hindshaker, a novel mutant showing hypomyelination preferentially affecting the spinal cord. Journal of Neurocytology 26, 557–566.
Klugmann, M., Schwab, M. H., P¬uhlhofer, A., Schneider, A., Zimmermann, F., Griffiths, I. R. & Nave, K.-A. (1997) Assembly of CNS myelin in the absence of proteolipid protein. Neuron 18, 59–70.
Knapp, P. E., Bartlett, W. P. & Skoff, R. P. (1987) Cultured oligodendrocytes mimic in vivo phenotypic characteristics: cell shape, expression of myelin-specific antigens and membrane production. Developmental Biology 120, 356–365.
Knapp, P. E. & Skoff, R. P. (1993) Jimpy mutation affects astrocytes: Lengthening of the cell cycle in vitro. Developmental Neuroscience 15, 31–36.
Knapp, P. E., Skoff, R. P. & Redstone, D. W. (1986) Oligodendroglial cell death in jimpy mice: an explanation for the myelin deficit. Journal of Neuroscience 6, 2813–2822.
Lachapelle, F. (1995) Glial transplants: An in vivo analysis of extrinsic and intrinsic determinants of dysmyelination in genetic variants. Brain Pathology 5, 289–299.
Lachapelle, F., Lapie, P. & Gumpel, M. (1992) Oligodendrocytes from jimpy and normal mature tissue can be ÔactivatedÕ when transplanted in a newborn environment. Developmental Neuroscience 14, 105–113.
Lachapelle, F., Lapie, P., Nussbaum, J. L. & Gumpel, M. (1990) Immunohistochemical studies on cross-transplantations between jimpy, shiverer and normal newborn mice. Journal of Neuroscience Research 27, 324–331.
Matsuda, Y., Koito, H. & Yamamoto, H. (1997) Induction of myelin-associated glycoprotein expression through neuron-oligodendrocyte contact. Developmental Brain Research 100, 110–116.
Mcphilemy, K., Griffiths, I. R., Mitchell, L. S. & Kennedy, P. G. E. (1991) Loss of axonal contact causes down-regulation of the PLP gene in oligodendrocytes: evidence from partial lesions of the optic nerve. Neuropathology and Applied Neurobiology 17, 275–287.
Mcphilemy, K., Mitchell, L. S., Griffiths, I. R., Morrison, S., Deary, A. W., Sommer, I. & Kennedy, P. G. E. (1990) Effect of optic nerve transection upon myelin protein gene expression by oligodendrocytes: evidence for axonal influences on gene expression. Journal of Neurocytology 19, 494–503.
Meier, C. & Bischoff, A. (1975) Oligodendroglial cell development in jimpy mice and controls.Anelectron microscopic study in the optic nerve. Journal of the Neurological Sciences 26, 517–528.
Nadon, N. L., Arnheiter, H. & Hudson, L. D. (1994) A combination of PLP and DM20 transgenes promotes partial myelination in the jimpy mouse. Journal of Neurochemistry 63, 822–833.
Nadon, N. L. & Duncan, I. D. (1995) Gene expression and oligodendrocyte development in the myelin deficient rat. Journal of Neuroscience Research 41, 96–104.
Nave, K.-A., Bloom, F. E. & Milner, R. J. (1987) A single nucleotide difference in the gene for myelin proteolipid protein defines the jimpy mutation in mouse. Journal of Neurochemistry 49, 1873–1877.
Nave, K.-A., Lai, C., Bloom, F. E. & Milner, R. J. (1986) Jimpy mutant mouse: A 74-base deletion in the mRNA for myelin proteolipid protein and evidence for a primary defect inRNAsplicing. Proceedings of the National Academy of Sciences USA 83, 9264–9268.
Nave, K.-A., Lai, C., Bloom, F. E. & Milner, R. J. (1987) Splice site selection in the proteolipid protein (PLP) gene transcript and primary structure of the DM-20 protein of central nervous system myelin. Proceedings of the National Academy of Sciences USA 84, 5665–5669.
Omlin, F. X. & Anders, J. J. (1983) Abnormal cell relationships in jimpy mice: electron microscopic and immunocytochemical findings. Journal of Neurocytology 12, 767–784.
Peyron, F., Timsit, S., Thomas, J. L., Kagawa, T., Ikenaka, K. & Zalc, B. (1997) In situ expression of PLP/DM-20,MBP, andCNPduring embryonic and postnatal development of the jimpy mutant and of transgenic mice overexpressing PLP. Journal of Neuroscience Research 50, 190–201.
Pfeiffer, S. E., Warrington, A. E. & Bansal, R. (1993) The oligodendrocyte and its many cellular processes. Trends in Cell Biology 3, 191–197.
Privat, A., Valat, J., Lachapelle, F., Baumann, N. & Fulcrand, J. (1982) Radioautographic evidence for the protracted proliferation of glial cells in the central nervous system of jimpy mice. Developmental Brain Research 2, 411–416.
Schneider, A., Griffiths, I. R., Readhead, C. & Nave, K.-A. (1995) Dominant-negative action of the jimpy mutation in mice complemented with an autosomal transgene for myelin proteolipid protein. Proceedings of the National Academy of Sciences USA 92, 4447–4451.
Seitelberger, F. (1995) Neuropathology and genetics of Pelizaeus-Merzbacher disease. Brain Pathology 5, 267–273.
Seitelberger, F., Urbanits, S., Nave, K.-A. (1996) Pelizaeus-Merzbacher disease. In Neurodystrophies and Neurolipidoses (edited by Moser, H. W.), pp. 559–579. Amsterdam: Elsevier Science.
Shiota, C., Ikenaka, K. & Mikoshiba, K. (1991) Developmental expression of myelin protein genes in dysmyelinating mutant mice: Analysis by nuclear run-off transcription assay, in situ hybridization, and immunohistochemistry. Journal of Neurochemistry 56, 818–826.
Skoff, R. P. (1976) Myelin deficit in the jimpy mouse may be due to cellular abnormalities in astroglia. Nature 264, 560–562.
Skoff, R. P. (1982) Increased proliferation of oligodendrocytes in the hypomyelinated mouse mutant-jimpy. Brain Research 248, 19–31.
Solly, S. K., Thomas, J. L., Monge, M., Demerens, C., Lubetzki, C., Gardinier, M. V., Matthieu, J. M. & Zalc, B. (1996) Myelin/oligodendrocyte glycoprotein (MOG) expression is associated with myelin deposition. Glia 18, 39–48.
Sorg, B. A., Agrawal, D., Agrawal, H. C. & Campagnoni, A. T. (1986) Expression of myelin proteolipid protein and basic protein in normal and dysmyelinating mutant mice. Journal of Neurochemistry 46, 379–387.
Sorg, B. A., Smith, M. M. & Campagnoni, A. T. (1987) Developmental expression of the myelin proteolipid protein and basic protein mRNA in normal and dysmyelinating mutant mice. Journal of Neurochemistry 49, 1146–1154.
Sturrock, R. R. (1983) Problems of glial identification and quantification in the ageing central nervous system. In Brain Ageing: Neuropathology and Neuropharmacology (edited by Cervos-Navarro, J. & Sarkander, H.-I.), pp. 179–209. New York: Raven Press.
Thomson, C. E., Montague, P., Jung, M., Nave, K.-A., Griffiths, I. R. & Nave, K. A. (1997) Phenotypic severity of murine Plp mutants reflects in vivo and in vitro variations in transport of PLP isoproteins. Glia 20, 322–332.
Timsit, S., Martinez, S., Allinquant, B., Peyron, F., Puelles, L. & Zalc, B. (1995) Oligodendrocytes originate in a restricted zone of the embryonic ventral neural tube defined by DM-20 mRNA expression. Journal of Neuroscience 15, 1012–1024.
Timsit, S. G., Bally-cuif, L., Colman, D. R. & Zalc, B. (1992) DM-20 mRNA is expressed during the embryonic development of the nervous system of the mouse. Journal of Neurochemistry 58, 1172–1175.
Trapp, B. D., Nishiyama, A., Cheng, D. & Macklin, E. (1997) Differentiation and death of premyelinating oligodendrocytes in developing rodent brain. Journal of Cell Biology 137, 459–468.
Vela, J. M., Dalmau, I., Gonz ´ alez, B. & Castellano, B. (1996) The microglial reaction in spinal cords of jimpy mice is related to apoptotic oligodendrocytes. Brain Research 712, 134–142.
Vela, J. M., Gonz ´ alez, B. & Castellano, B. (1998) Understanding glial abnormalities associated with myelin deficiency in the jimpy mutant mouse. Brain Research Reviews 26, 29–42.
Verity, A. N., Levine, M. S. & Campagnoni, A. T. (1990) Gene expression in the jimpy mutant: evidence for fewer oligodendrocytes expressing myelin protein genes and impaired translocation of myelin basic protein mRNA. Developmental Neuroscience 12, 359–372.
Wang, S. L., Sdrulla, A. D., Disibio, G., Bush, G., Nofziger, D., Hicks, C., Weinmaster, G. & Barres, B. A. (1998) Notch receptor activation inhibits oligodendrocyte differentiation. Neuron 21, 63–75.
Williams, M. A. (1977) Stereological techniques. In Practical Methods in Electron Microscopy. Vol. 6: Quantitative Methods in Biology (edited by Glauert, A. M.), pp. 5–84. Amsterdam: North Holland.
Yanagisawa, K. & Quarles, R. H. (1986) Jimpy mice: quantitation of myelin-associated glycoprotein and other proteins. Journal of Neurochemistry 47, 322–325.
Yu, W.-P., Collarini, E. J., Pringle, N. P. & Richardson, W. D. (1994) Embryonic expression of myelin genes: Evidence for a focal source of oligodendrocyte precursors in the ventricular zone of the neural tube. Neuron 12, 1353–1362.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Thomson, C., Anderson, T., McCulloch, M. et al. The early phenotype associated with the jimpy mutation of the proteolipid protein gene. J Neurocytol 28, 207–221 (1999). https://doi.org/10.1023/A:1007024022663
Issue Date:
DOI: https://doi.org/10.1023/A:1007024022663