Abstract
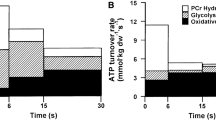
Coupling of ATP-generating with ATP-consuming processes is an essential component in the cardiac bioenergetics responsible for optimal myocardial function. Although a number of enzymatic systems have been implicated in securing proper intracellular energy communication, their integrative response in a failing myocardium has not been determined so far. Therefore, we measured catalytic activities of enzymes responsible for the communication between ATP-generating and ATP-consuming processes in ventricular samples obtained from normal dogs and dogs with tachycardia-induced heart failure. In the failing myocardium, phosphotransfer activities of creatine kinase, adenylate kinase, 3-phosphoglycerate kinase and pyruvate kinase, which collectively deliver ATP and remove ADP from myofibrillar ATPases, were depressed by 30, 21, 44 and 20%, respectively, when compared to normal controls. The activity of hexokinase, an enzyme which directs phosphoryls into the glycolytic phosphotransfer pathway, was unchanged. Also, the activity of glyceraldehyde-3-phosphate dehydrogenase, which may shuttle inorganic phosphate between ATPases and ATP-synthases, was not affected by heart failure. However, the CO2-hydration activity of carbonic anhydrase, which together with creatine kinase, is presumed responsible for removal of protons from ATPases, was diminished by 21%. As these enzymatic systems are collectively required for adequate delivery of high-energy phosphoryl to, and removal of end-products from, cellular ATPases, the cumulative deficit in their flux capacities may provide a bioenergetic basis for impaired contraction-relaxation in the failing heart.
Similar content being viewed by others
References
Ingwall JS, Nascimben L, Gwathmey JK: Heart failure: is the pathology due to calcium overload or to mismatch in the energy supply and demand? In: JK Gwathmey, GM Briggs and PD Allen (eds). Heart Failure: Basic Science and Clinical Aspects. Marcel Dekker, New York, 1993, pp 667–700
Saks VA, Tiivel T, Kay L, Novel-Chate V, Daneshrad Z, Rossi A, Fontaine E, Keriel C, Leverve X, Ventura-Clapier R, Anflous K, Samuel JL, Rappaport L: On the regulation of cellular energetics in health and disease. Mol Cell Biochem 160–161: 195–208, 1996
Cohn JN, Bristow MR, Chien KR, Colucci WS, Frazier OH, Leinwand LA, Lorell BH, Moss AJ, Sonnenblick EH, Walsh RA, Mockrin SC, Reinlib L: Report of the National Heart, Lung, and Blood Institute Special Emphasis Panel on Heart Failure Research. Circulation 95: 766–70, 1997
Rauch U, Schulze K, Witzenbichler B, Schultheiss HP: Alteration of the cytosolic-mitochondrial distribution of high-energy phosphates during global myocardial ischemia may contribute to early contractile failure. Circ Res 75: 760–769, 1994
Nascimben L, Ingwall JS, Pauletto P, Friedrich J, Gwathmey JK, Saks V, Pessina AC, Allen PD: Creatine kinase system in failing and nonfailing human myocardium. Circulation 94: 1894–1901, 1996
Lewandowski ED, Yu X, LaNoue KF, White LT, Doumen C, O'Donnell JM: Altered metabolite exchange between subcellular compartments in intact postischemic rabbit hearts. Circ Res 81: 165–175, 1997
Zuurbier CJ, van Beek JH: Mitochondrial response to heart rate steps in isolated rabbit heart is slowed after myocardial stunning. Circ Res 81: 69–75, 1997
van Deursen J, Heerschap A, Oerlemans F, Ruitenbeek W, Jap P, ter Laak H, Wieringa B: Skeletal muscle of mice deficient in muscle creatine kinase lack burst activity. Cell 74: 621–631, 1993
Veksler VI, Kuznetsov AV, Anflous K, Mateo P, van Deursen J, Wieringa B, Ventura-Clapier R: Muscle creatine kinase-deficient mice: Cardiac and skeletal muscles exhibit tissue-specific adaptation of the mitochondrial function. J Biol Chem 270: 19921–19929, 1995
Ventura-Clapier R, Kuznetsov AV, d'Albis A, van Deursen J, Wieringa B, Veksler VI: Muscle creatine kinase-deficient mice: Alterations in myofibrillar function. J Biol Chem 270: 19914–19920, 1995
Steeghs K, Benders A, Oerlemans F, de Haan A, Heerschap A, Ruitenbeek W, Jost C, van Deursen J, Perryman B, Pette D, Bruckwilder M, Koudijs J, Jap P, Veerkamp J, Wieringa B: Altered Ca2+ responses in muscles with combined mitochondrial and cytosolic creatine kinase deficiencies. Cell 89: 93–103, 1997
Steeghs K, Oerlemans F, Dehaan A, Heerschap A, Verdoodt L, Debie M, Ruitenbeek W, Benders A, Jost C, Vandeursen J, Tullson P, Terjung R, Jap P, Jacob W, Pette D, Wieringa B: Cytoarchitectural and metabolic adaptations in muscles with mitochondrial and cytosolic creatine kinase deficiencies. Mol Cell Biochem 184: 183–194, 1998
Dzeja PP, Zeleznikar RJ, Goldberg ND: Adenylate kinase: Kinetic behavior in intact cells indicates it is integral to multiple cellular processes. Mol Cell Biochem 84: 169–182, 1998
Dzeja PP, Kalvenas A, Toleikis A, Praskevicius A: The effect of adenylate kinase activity on the rate and efficiency of energy transport from mitochondria to hexokinase. Biochem Int 10: 259–265, 1985
Savabi F: Interaction of creatine kinase and adenylate kinase systems in muscle cells. Mol Cell Biochem 133–134: 145–152, 1994
Elvir-Mairena JR, Jovanovic A, Gomez LA, Alekseev AE, Terzic A: Reversal of the ATP-liganded state of ATP-sensitive K+ channels by adenylate kinase activity. J Biol Chem 271: 31903–31908, 1996
van Beek JHGM, Tian X, Zuurbier CJ, Degroot B, Vanechteld CA, Eijgelshoven MHJ, Hak JB: The dynamic regulation of mitochondrial oxidative phosphorylation-analysis of the response time of oxygen consumption. Mol Cell Biochem 184: 321–344, 1998
Dzeja PP, Zeleznikar RJ, Goldberg ND: Suppression of creatine kinase-catalyzed phosphotransfer results in increased phosphoryl transfer by adenylate kinase in intact skeletal muscle. J Biol Chem 271: 12847–12851, 1996
Nicolay K, Vandorsten FA, Reese T, Kruiskamp MJ, Gellerich JF, Vanechteld CJA: In situ measurements of creatine kinase flux by NMR – the lessons from bioengineered mice. Mol Cell Biochem 184: 195–208, 1998
Saupe KW, Spindler M, Tian R, Ingwall JS: Impaired cardiac energetics in mice lacking muscle-specific isoenzymes of creatine kinase. Circ Res 82: 898–907, 1998
Tian R, Christe ME, Spindler M, Hopkins JC, Halow J, Camacho SA, Ingwall JS: Role of MgADP in the development of diastolic dysfunction in the intact beating rat heart. J Clin Invest 99: 745–751, 1997
He M-X, Wang S, Downey HF: Correlation between myocardial contractile force and cytosolic inorganic phosphate during early ischemia. Am J Physiol 272: H1333–H1341, 1997
Saks VA, Khuchua ZA, Vasilyeva EV, Belikova OY, Kuznetsov AV: Metabolic compartmentation and substrate channeling in muscle cells. Role of coupled creatine kinases in in vivo regulation of cellular respiration – a synthesis. Mol Cell Biochem 133–134: 155–192, 1994
Jacobus WE: Theoretical support for the heart phosphocreatine energy transport shuttle based on the intracellular diffusion limited mobility of ADP. Biochem Biophys Res Commun 133: 1035–1041, 1985
Toleikis AI, Dzeja PP, Praskevicius AK, Januskevicius Z: Functional state and the mechanisms of mitochondrial injury of ischemic heart. Sov Med Rev A Cardiol 2: 95–132, 1989
Dzeja PP, Vitkevicius KT, Redfield MM, Burnett JC, Terzic A: Adenylate kinase catalyzed phosphotransfer in the myocardium: Increased contribution in heart failure. Circ Res 84: 1137–1143, 1999
Kingsley-Hickman PB, Sako EY, Mohanakrishnan P, Robitaille PML, From AHL, Foker JE, Ugurbil K: 31P NMR studies of ATP synthesis and hydrolysis kinetics in the intact myocardium. Biochemistry 26: 7501–7510, 1987
Dzeja PP, Terzic A: Phosphotransfer reactions in the regulation of ATP-sensitive K+ channels. FASEB J 12: 523–529, 1998
Wallimann T, Wyss M, Brdiczka D, Nicolay K, Eppenberger HM: Intracellular compartmentation, structure and function of creatine kinase isoenzymes in tissues with high and fluctuating energy demands: The ‘phosphocreatine circuit’ for cellular energy homeostasis. Biochem J 28: 21–40, 1992
Gros G, Moll W, Hoppe H, Gros H: Proton transport by phosphate diffusion – A mechanism of facilitated CO2 transfer. J Gen Physiol 67: 773–790, 1976
Pierce GN, Philipson KD: Binding of glycolytic enzymes to cardiac sarcolemmal and sarcoplasmic reticular membranes. J Biol Chem 260: 6862–6870, 1985
Wegmann G, Zanolla E, Eppenberger HM, Wallimann T: In situ compartmentation of creatine kinase in intact sarcomeric muscle: The acto-myosin overlap zone as a molecular sieve. J Muscle Res Cell Motil 13: 420–435, 1992
Bruns W, Gros G: Membrane-bound carbonic anhydrase in the heart. Am J Physiol 262: H577–H584, 1992
Yamamoto K, Burnett JC, Meyer LM, Sinclair L, Stevens TL, Redfield MM: Ventricular remodeling during development and recovery from modified tachycardia-induced cardiomyopathy model. Am J Physiol 271: R1529–R1534, 1996
Yamamoto K, Burnett JC, Redfield MM: Effect of endogenous natriuretic peptide system on ventricular and coronary function in failing heart. Am J Physiol 273: H2406–H2414, 1997
Raff GL, Glantz SA: Volume leading slows left ventricular isovolumic relaxation rate. Circ Res 48: 813–824, 1981
Weisfeldt ML, Frederiksen JW, Yin FC, Weiss JL: Evidence of incomplete left ventricular relaxation in the dog: prediction from the time constant for isovolumic pressure fall. J Clin Invest 62: 1296–1302, 1978
Brindle KM, Radda GK: 31P-NMR saturation transfer measurements of exchange between Pi and ATP in the reactions catalysed by glyceraldehyde-3-phosphate dehydrogenase and phosphoglycerate kinase in vitro. Biochim Biophys Acta 928: 45–55, 1987
Knight RJ, Kofoed KF, Schelbert HR, Buxton DB: Inhibition of glyceraldehyde-3-phosphate dehydrogenase in post-ischaemic myocardium. Cardiovasc Res 32: 1016–1023, 1996
Fremont P, Boudriau S, Tremblay RR, Cote C, Rogers PA: Acetazolamide-sensitive and resistant carbonic anhydrase activity in rat and rabbit skeletal muscles of different fiber type composition. Int J Biochem 21: 143–147, 1989
Ottaway JH, Mowbray J: The role of compartmentation in the control of glycolysis. Curr Top Cell Reg 12: 107–208, 1977
Bessman SP, Geiger PJ: Compartmentation of hexokinase and creatine phosphokinase, cellular regulation, and insulin action. Curr Top Cell Reg 16: 55–86, 1980
Terzic A, Puceat M, Clement O, Scamps F, Vassort G. Alpha1-adrenergic effects on intracellular pH and calcium and on myofilaments in single rat cardiac cells. J Physiol 447: 275–292, 1992
Liedtke AJ, Nellis SH, Neely JR, Hughes HC: Effects of treatment with pyruvate and tromethamine in experimental myocardial ischemia. Circ Res 39: 378–387, 1976
Wetzel P, Liebner T, Gros G: Carbonic anhydrase inhibition and calcium transients in soleus fibers. FEBS Lett 267: 66–70, 1990
Zeleznikar RJ, Dzeja PP, Goldberg ND: Adenylate kinase-catalyzed phosphoryl transfer couples ATP utilization with its generation by glycolysis in intact muscle. J Biol Chem 270: 7311–7319, 1995
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Dzeja, P.P., Pucar, D., Redfield, M.M. et al. Reduced activity of enzymes coupling ATP-generating with ATP-consuming processes in the failing myocardium. Mol Cell Biochem 201, 33–40 (1999). https://doi.org/10.1023/A:1007016703229
Issue Date:
DOI: https://doi.org/10.1023/A:1007016703229