Abstract
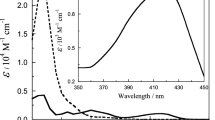
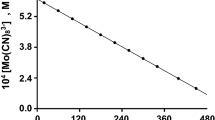
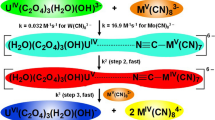
The kinetics of oxidation of the neutralized α-hydroxy acids: lactic, α-hydroxyisobutyric, mandelic, benzilic and atrolactic acids by tris(pyridine-2-carboxylato)manganese(III) have been studied. The reactions were carried out in a Na(pic)-picH [Na(pic) = sodium salt of pyridine-2-carboxylic acid and picH = pyridine-2-carboxylic acid] buffer medium in the 4.89–6.10pH range. The oxidation rate was found to be independent of pH, and rate follows the order: benzilate > mandelate >atrolactate>lactate > α-hydroxy isobutyrate. The oxidation products are MeCHO, Me2CO, PhCHO, Ph2CO and PhCOMe for the respective reactions. A mechanism is proposed involving intermediate formation of hepta-coordinated MnIII complexes in a fast step. The complexes then decompose to give free radicals and MnII in the rate determining step. The free radicals subsequently react with another molecule of the MnIII species to give the respective carbonyl compounds in a fast step.
Similar content being viewed by others
References
K. K. Sen Gupta, S. Maiti, U. Chatterjee and T. Samanta, Transition Met. Chem., 7, 89 (1982).
P. Levesley and W. A. Waters, J. Chem. Soc., 217 (1955).
K. K. Sen Gupta, A. Banerjee and H. Chatterjee, Tetrahedron, 48, 5323 (1992).
J. R. Jones, W. A. Waters and J. S. Littler, J. Chem. Soc., 630 (1961).
B. Krishna and K. C. Tewari, J. Chem. Soc., 3097 (1961).
A. McAuley, J. Chem. Soc., 4054 (1965).
S. Prasad and J. Choudhary, Ind. J. Chem., 17A, 167 (1979).
S. B. Hanna and S. A. Sarac, J. Org. Chem., 42, 2063 (1977).
S. B. Hanna and S. A. Sarac, J. Org. Chem., 42, 2069 (1977).
K. K. Sen Gupta and T. Sarkar, Tetrahedron, 31, 123 (1975).
K. K. Sen Gupta, A. K. Chatterjee and J. K. Chakladar, Ind. J. Chem., 10, 493 (1972).
B. N. Figgis, C. L. Raston, R. P. Sharma and A. H. White, Aust. J. Chem., 31, 2545 (1978).
R. C. Weast, C. R. C. Handbook of Chemistry and Physics, 66th Edit., CRC Press, USA, 1986 p. D 162.
S. Ghosh, P. K. Ray, T. K. Bandyopadhyay and A. K. Deb, Z. Naturforsch, 36b, 1270 (1981).
M. M. Ray, J. N. Adhya, D. Biswas and S. N. Poddar, Aust. J. Chem., 19, 1737 (1966).
A. I. Vogel, Textbook of Quantitative Inorganic Analysis, 4th Edit., ELBS, Longman, London, 1986, pp. 348–349.
F. Feigl, V. Anger and R. Oesper, Spot Tests in Organic Analysis, 7th Edit., Elsevier, New York, 1966, p. 387.
E. H. Huntress and S. P. Mulliken, Identification of Pure Organic Compounds, Wiley, Inc. New York, Fourth Printing, 1953, pp. 50, 44, 374, 388, 60, 363, 610.
R. T. Morrison and R. N. Boyd, Organic Chemistry, Allyn and Bacon, Boston, 1962, p. 715
G. St. Nikolov, Inorg. Chim. Acta, 5, 559 (1971).
O. Exner, Nature, 201, 488 (1964).
J. E. Leffer, J. Org. Chem., 20, 1202 (1955).
W. A. Waters, J. R. Jones and J. S. Littler, J. Chem. Soc., 240 (1961).
T. J. Kemp and W. A. Waters, J. Chem. Soc., 1192 (1964).
F. A. Westheimer, Chem. Rev., 45, 419 (1949).
G. V. Bakore and S. Narain, J. Chem. Soc., 3419 (1963).
S. Richards, B. Pedersen, J. V. Silverton and J. L. Hoard, Inorg. Chem., 3, 27 (1964).
R. E. Hamm and M. A. Suwyn, Inorg. Chem., 6, 139 (1967).
G. Wilkinson, R. D. Gillard and J. A. McCleverty, Comprehensive Coordination Chemistry, Pergamon Press, Oxford, 1st Edit., 1987, Vol. 4, p. 64.
S. Kundu, A. K. Bhattacharya and R. Banerjee, J. Chem. Soc., Dalton Trans., 3952 (1996).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gupta, K.K.S., Bhattacharjee, N., Pal, B. et al. Kinetics and mechanism of the oxidation of neutralized α-hydroxy acids by tris(pyridine-2-carboxylato)manganese(III). Transition Metal Chemistry 24, 268–273 (1999). https://doi.org/10.1023/A:1006989810426
Issue Date:
DOI: https://doi.org/10.1023/A:1006989810426