Abstract
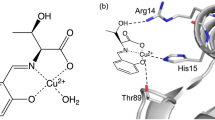
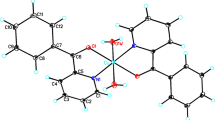
Reaction of Cu(ClO4)2·6H2O with 1-methylbenzotriazole (Mebta) in EtOH yields [Cu(Mebta)4(H2O)] (ClO4)2·0.4EtOH in ca. 75% yield. The structure of this salt has been determined by single-crystal X-ray crystallography. Mebta behaves as a monodentate ligand binding through N(3). The metal coordination geometry is best described as distorted square pyramidal with the H2O ligand occupying the apical site. The complex was also characterized by molar conductivity, room-temperature effective magnetic moment and spectroscopic (i.r., far-i.r., u.v./vis, e.s.r.) studies. The data are discussed in terms of the nature of bonding and known structure. Comparison between the structural and spectroscopic properties of [Cu(Mebta)4(H2O)](ClO4)2·0.4EtOH and those of the CuII site of Cu–Zn superoxide dismutase shows that the former can be considered as a fairly good model for the latter.
Similar content being viewed by others
References
J. Selverstone Valentine in I. Bertini, H.B. Gray, S.J. Lippard and J. Selverstone Valentine (eds), Bioinorganic Chemistry, University Science Books, Mill Valley, California, 1994, p. 298.
S.J. Lippard and J.M. Berg, Principles of Bioinorganic Chemistry, University Science Books, Mill Valley, California, 1994, p. 325.
J.L. Pierre, P. Chautemps, S. Refaif, C. Beguin, A. El Marzouki, G. Serratrice, E. Saint-Aman and P. Rey, J. Am. Chem. Soc., 117, 1965 (1995) and refs cited therein.
H.J. Scholl, J. Hx00FC;ttermann and M.S. Viezzoli, Inorg. Chim. Acta, 273, 131 (1998) and refs cited therein.
I. Fridovich, Ann. Rev. Biochem., 44, 147 (1975).
J.A. Tainer, E.D. Getzoff, J.S. Richardson and D.C. Richardson, Nature, 306, 284 (1983).
D.F. Xiang, X.S. Tan, Q.W. Hang, W.X. Tang, B.M. Wu and T.C.W. Mak, Inorg. Chim. Acta, 277, 21 (1998).
W.H. Koppenol, Nature, 262, 420 (1976).
J.M. McCord, S.H. Syokes and K. Wong, Adv. In¯amm. Res., 1, 273 (1979).
B. Halliwell and J.M.C. Gutteridge, J. Biochem., 219, 1 (1984).
B. Halliwell and J.M.C. Gutteridge, Arch. Biochem. Biophys., 246, 501 (1986).
L.W. Oberley and G.R. Buettner, Cancer Res., 39, 1141 (1979).
J.M. McCord, Science, 185, 529 (1974).
D.F. Xiang, C.Y. Duan, X.S. Tan, Q.W. Hang and W.X. Tang, J. Chem. Soc., Dalton Trans., 1201 (1998) and refs cited therein.
J.R.J. Sorenson, Prog. Med. Chem., 26, 437 (1989).
Q. Lu, Q.H. Luo, A.B. Dai, Z.Y. Zhou and G.Z. Hu, J. Chem. Soc.,Chem. Commun., 1429 (1990) and refs. therein.
M. Zongwan, C. Dong, T. Wenxia, Y. Kaibei and L. Li, Polyhedron, 11, 191 (1992).
R. Bhalla, M. Helliwell and C.D. Garner, Inorg. Chem., 36, 2944 (1997).
M. Linss and U. Weser, Inorg. Chim. Acta, 125, 117 (1986).
Q. Luo, Q. Lu, A. Dai and L. Huang, J. Inorg. Biochem., 51, 655 (1993).
Q. Lu, C.Y. Shen and Q.H. Luo, Polyhedron, 12, 2005 (1993).
J. Mx00FC;ller, K. Felix, C. Maichle, E. Lengfelder, J. Strähle and U. Weser, Inorg. Chim. Acta, 233, 11 (1995).
C.M. Liu, R.G. Xiong, X.Z. You and Y.J. Liu, Polyhedron, 15, 4565 (1996).
C.M. Liu, R.G. Xiong, X.Z. You, H.K. Fun and K. Sivakumar, Polyhedron, 16, 119 (1997).
J.C. Plakatouras, D. Mentzafos, A. Terzis, T. Bakas, V. Papaefthymiou and S.P. Perlepes, Polyhedron, 11, 2657 (1992).
D. Kovala-Demertzi and S.P. Perlepes, Transition Met. Chem., 19, 7 (1994).
E. Diamantopoulou, Th. F. Zafiropoulous, S.P. Perlepes, C.P. Raptopoulou and A. Terzis, Polyhedron, 13, 1593 (1994).
J.C. Plakatouras, T. Bakas, C.J. Huffman, J.C. Huffman, V. Papaefthymiou and S.P. Perlepes, J. Chem. Soc., Dalton Trans., 2737 (1994).
K. Skorda, E.G. Bakalbassis, J. Mrozinski, S.P. Perlepes, C.P. Raptopoulou and A. Terzis, J. Chem. Soc., Dalton Trans., 2317 (1995).
E.G. Bakalbassis, E. Diamantopoulou, S.P. Perlepes, C.P. Raptopoulou, V. Tangoulis, A. Terzis and Th.F. Zafiropoulous, J. Chem. Soc., Chem. Commun., 1347 (1995).
V. Tangoulis, C.P. Raptopoulou, A. Terzis, E.G. Bakalbassis, E. Diamantopoulou and S.P. Perlepes, Inorg. Chem., 37, 3142 (1998).
G.M. Sheldrick. SHELXS-86. Program for the solution of crystal structures, University of GoÈ ttingen, Germany, 1986.
G.M. Sheldrick. SHELXL-93. Program for crystal structure refinement, University of GoÈ ttingen, Germany, 1993.
S.P. Perlepes, J.C. Huffman and G. Christou, Polyhedron, 11, 1471 (1992).
A.W. Addison, T.N. Rao, J. Reedijk, J. Rijn and G.C. Verschoor, J. Chem. Soc., Dalton Trans., 1349 (1984).
I. Bertini, L. Banci and M. Piccioli, Coord. Chem. Rev., 100, 67 (1990).
W.J. Geary, Coord. Chem. Rev., 7, 81 (1971).
L.S. Gelfand, F.J. Iaconiani, L.L. Pytlewski, A.N. Speca, C.M. Mikulski and N.M. Karayannis, J. Inorg. Nucl. Chem., 42, 377 (1980).
K. Nakamoto, Infrared and Raman Spectra of Inorganic and Coordination Compounds, 4th edn., Wiley, New York, 1986, pp. 139, 227±230, 251, 253.
J. Reedijk, A.R. Siedle, R.A. Velapoldi and J.A.M. Van Hest, Inorg. Chim. Acta, 74, 109 (1983).
B. Slootmaekers, S.P. Perlepes and H.O. Desseyn, Spectrochim. Acta, 45A, 1211 (1989).
P. Jacobs, K. Dimitrou, S.P. Perlepes, J. Plakatouras and H.O. Desseyn, Bull. Soc. Chim. Belg., 98, 901 (1989).
C. Vansant, H.O. Desseyn, V. Tangoulis, C.P. Raptopoulou, A. Terzis and S.P. Perlepes, Polyhedron, 14, 2115 (1995).
R.J.H. Clark and C.S. Williams, Inorg. Chem., 4, 350 (1965).
W.R. McWhinnie, J.Inorg. Nucl. Chem., 27, 1063 (1965).
A.B.P. Lever and E. Mantovani, Inorg. Chem., 10, 817 (1971).
F.A. Cotton and G. Wilkinson, Advanced Inorganic Chemistry, 5th edn., Wiley, New York, 1988, p. 768.
M. Palaniandavar, R.J. Butcher and A.W. Addison, Inorg. Chem., 35, 467 (1996).
A.B.P. Lever, Inorganic Electronic Spectroscopy, 2nd Edit., Elsevier, Amsterdam, 1984, pp. 554±567.
H.W. Richardson, J.R. Wasson, W.E. Estes and W.E. Hatfield, Inorg. Chim. Acta, 23, 205 (1977)
B.J. Hathaway, J. Chem. Soc., Dalton Trans., 1196 (1972).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Skorda, K., Perlepes, S.P., Raptopoulou, C.P. et al. A structural model for the copper(II) site of Cu-Zn superoxide dismutase: preparation, crystal structure and properties of [Cu(Mebta)4(H2O)](ClO4)2·0.4EtOH (Mebta = 1-methylbenzotriazole). Transition Metal Chemistry 24, 541–545 (1999). https://doi.org/10.1023/A:1006988106184
Issue Date:
DOI: https://doi.org/10.1023/A:1006988106184