Abstract
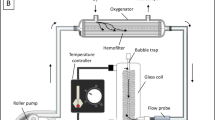
Numerous studies have focused on the metabolic contributions of glucose and other substrates in isolated tissue preparations by examining the effects of eliminating glucose from the physiologic perfusate or bath solution. To date, however, an effective method of glucose removal from the blood supply to selected tissue in the whole animal model has not been available. We have developed a method for blood glucose removal by continuous flow dialysis. This method was used to generate isolated coronary hypoglycemia for an investigation of myocardial metabolic substrate selection during hypoperfusion in open-chest, anesthetized dogs. Arterial blood was passed through the dialysis system against an isotonic and physiologic dialysate solution prior to controlled coronary perfusion. During normal perfusion pressure (100 mmHg), with a coronary blood flow of 32 ± 4 ml/min, arterial blood glucose was reduced from 3.26 ± 0.31 to 0.54 ± 0.14 mM. When blood flow was reduced to 12 ± 3 ml/min with lower perfusion pressure (40 mmHg), dialysis reduced arterial glucose from 3.53 ± 0.36 to 0.15 ± 0.03 mM. We conclude that this is an effective method for producing regional hypoglycemia.
Similar content being viewed by others
References
Donoso P, Mill A, O'Neill SC, Eisner DA: Fluorescence measurements of cytoplasmic and mitochondrial sodium concentration in rat ventricular myocytes. J Physiol 448: 493–509, 1992
Oliva EA, Savino EA: Effects of 2-deoxy-D-glucose on the contraction frequency of the isolated atria from fed and fasted rats. Arch Int Physiol Biochim Biophys 87: 59–63, 1979
Pacini DJ, Kane KA: Effects of components of myocardial ischemia on cardiac action potentials in vitro. J Cardiovasc Pharmacol 18: 261–266, 1991
Ruiz RD, Fiol de Cuneo M, Lacuara JL, Santillan de Torres R: Influence of the metabolic environment on oxygen uptake and isometric developed tension of isolated rat portal vein. Acta Physiol Pharmacol Ther Latinoam 35: 459–466, 1985
Scott DP, Coburn RF: Phosphocreatine and oxidative metabolismcontraction coupling in rabbit aorta. Am J Physiol 257: H597–H602, 1989
Crepel V, Krnjevic K, Ben-Ari Y: Developmental and regional differences in the vulnerability of rat hippocampal slices to lack of glucose. Neuroscience 47: 579–587, 1992
Duchen MR: Effects of metabolic inhibition on the membrane properties of isolated mouse primary sensory neurones. J Physiol 424: 387–409, 1990
Duchen MR, Valdeolmillos M, O'Neill SC, Eisner DA: Effects of metabolic blockade on the regulation of intracellular calcium in dissociated mouse sensory neurones. J Physiol 424: 411–426, 1990
Joris L, Quinton PM: Evidence for electrogenic Na-glucose cotransport in tracheal epithelium. Pflügers Arch 415: 118–120, 1989
Rose CR, Waxman SG, Ransom BR: Effects of glucose deprivation, chemical hypoxia, and simulated ischemia on Na+ homeostasis in rat spinal cord astrocytes. J Neurosci 18: 3554–3562, 1998
Schurr A, Payne RS, Miller JJ, Rigor BM: Brain lactate, not glucose, fuels the recovery of synaptic function from hypoxia upon reoxygenation: An in vitro study. Brain Res 744: 105–111, 1997
Abe M, Furukawa T: Spontaneous rhythmicity induced by glucose removal in the guinea-pig tracheal smooth muscle. Arch Int Pharmacodyn Ther 261: 51–58, 1983.
Ashoori F, Takai A, Tokuno H, Tomita T: Effects of glucose removal and readmission on potassium contracture in the guinea-pig taenia coli. J Physiol 356: 33–48, 1984
Bose D, Bose R: Mechanics of guinea pig taenia coli smooth muscle during anoxia and rigor. Am J Physiol 229: 324–328, 1975
Chastre E, Enami S, Rosselin G, Gespach C: Vasoactive intestinal peptide receptor activity during enterocyte-like differentiation and retrodifferentiation of the human colonic cancerous subclone HT29–18. FEBS Lett 188: 197–204, 1985
Nasu T, Baba K: Mechanics of manganese ions-induced contraction of ileal longitudinal muscle in Ca2+-free, high-K+ medium. Gen Pharmacol 28: 503–507, 1997
Shimizu K, Hori M, Mitsui M, Hayashi Y, Nakajyo S, Urakawa N: The properties of a high K+-induced contraction in the ileal longitudinal and circular muscles of cat. Nippon Keikatsukin Gakkai Zasshi 23: 47–53, 1987
Fraser LR, Quinn PJ: A glycolytic product is obligatory for initiation of the sperm acrosome reaction and whiplash motility required for fertilization in the mouse. J Reprod Fertil 61: 25–35, 1981
Sunano S: Comparison of effects of low temperature and metabolic inhibition on high-K induced contracture of vas deferens. Arch Int Pharmacodyn Ther 248: 33–42, 1980
Kitzman HH Jr, McMahon RJ, Williams MG, Frost SC: Effect of glucose deprivation on GLUT-1 expression in 3T3-L1 adipocytes. J Biol Chem 268: 1320–1325, 1993
In't Veld PA, Pipeleers DG, Gepts W: Glucose alters configuration of gap junctions between pancreatic islet cells. Am J Physiol 251: C191–C196, 1986
Lopaschuk GD, Belke DD, Gamble J, Itoi T, Schonekess BO: Regulation of fatty acid oxidation in the mammalian heart in health and disease. Biochim Biophys Acta 1213: 263–276, 1994
Stantey WC, Lopaschuk GD, Hall JL, McCormack JG: Regulation of myocardial metabolism under normal and ischaemic conditions. Cardiovasc Res 33: 243–257, 1997
Van der Vusse GJ, Glatz JFC, Stam HCG, Reneman RS: Fatty acid homeostasis in the normoxic and ischemic heart. Physiol Rev 72: 881–940, 1992
Taegtmeyer H, Goodwin GW, Doenst T, Frazier OH: Substrate metabolism as a determinant for postischemic functional recovery of the heart. Am J Cardiol 80: 3A–10A, 1997
Rhoades RA, Tanner GA: Medical Physiology. Little, Brown and Company, 1995, p 211
Hart BJ: The effects of hyperlipidemia and hypoglycemia on myocardial contractile function and oxygen utilization during coronary hypoperfusion. Unpublished master's thesis, University of North Texas Health Science Center at Fort Worth, Fort Worth, TX, 1998
Persson E, Nordenstrom J, Nilsson-Ehle P: Plasma kinetics of lipoprotein lipase and hepatic lipase activities induced by heparin and a low molecular weight heparin fragment. Scand J Clin Lab Invest 47: 151–155, 1987
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hart, B.J., Bian, X., Williams Jr, A.G. et al. A method for producing regional hypoglycemia in blood perfused tissue. Mol Cell Biochem 200, 177–181 (1999). https://doi.org/10.1023/A:1006980419023
Issue Date:
DOI: https://doi.org/10.1023/A:1006980419023