Abstract
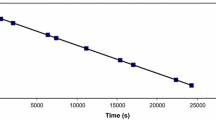
The complexes [Co(tetren)dmf](ClO4)3 and [Co(tetren)-dmso](ClO4)3 have been prepared from alpha alpha-[Co(tetren)-Cl](ClO4)Cl (tetren=1,11-diamino-3,6,9-triazaundecane). 1H-n.m.r. and i.r. measurements confirm that the complexes contain O-bonded dmf and dmso. A biphasic reaction is observed in the base hydrolysis of the dmf derivative, monitored by the pH-stat method, with the fast reaction having kOH=1.2*104dm3mol-1s-1 and the slower reaction kOH=1.9*102dm3mol-1s-1 at 25degC and I=0.1moldm3. The fast reaction is assigned to the hydrolysis of the alpha beta(R)-[Co(tetren)dmf]3+ and the slower reaction to that of the alpha The reaction appears to proceed predominantly by a DCB pathway without parallel hydrolysis of coordinated dmf, which has been observed in the hydrolysis of [Co-(NH3)5dmf]3+. Base hydrolysis of [Co(tetren)dmso]3+ was monitored spectrophotometrically over the pH range 4.2 to 5.0. A single reaction was observed with kOH=1.9*106dm3mol-1 s-1 at 25°C and I=0.1 moldm-3. The rapid base hydrolysis is attributed to hydrolysis of the alpha beta(R)- or the alpha beta(S)-[Co(tetren)-dmso]3+ isomer rather than the alpha alpha-isomer.
Similar content being viewed by others
References
N. L. Allinger and E. Eliel, Topics in Stereochemistry, Wiley Interscience, New York, 1971, Vol. 6, p. 219.
M. R. Snow, D. A. Buckingham, P. A. Marzilli and A. M. Sargeson, Chem. Commun., 891 (1969).
M. R. Snow, J. Am. Chem. Soc., 92, 3610 (1970). It is interesting to compare the structure of αα-[Co(tetren)Cl] (ClO4)Cl determined by Snow with our recent crystal structure of this compound. Both crystals are monoclinic but Snow's compound has the cell constants a = 12.31 Å, b = 11.20 Å, c = 12.67 Å, β = 119.3° and V = 1637.8 Å3. The crystals examined by us had the cell constants a = 9.85 Å, b = 13.80 Å, c = 13.91 Å, β = 111.9° and V = 1620 Å3. A theoretical gas phase structure calculated for the αα-isomer shows the complex to have mirror symmetry; however, the crystal examined by Snow was found to have the mirror symmetry destroyed by an envelope conformation of the chelate ring (IV), which was attributed to crystal packing forces. The crystal structure determined here is much closer to the gas phase structure with chelate ring (IV) having a gauche conformation.
C. W. Davies, J. Chem. Soc., 2093 (1938).
H. S. Harned and R. A. Robinson, Trans. Faraday Soc., 36, 973 (1940).
R. W. Hay and P. L. Cropp, J. Chem. Soc. (A), 42, (1969)
D. A. Buckingham, J. MacB. Harrowfield and A. M. Sargeson, J. Am. Chem. Soc., 96, 1726 (1974).
R. W. Hay and R. Bembi, Inorg. Chim. Acta, 64, L199 (1982).
R. W. Hay and F. McLaren, unpublished observations.
C. R. Piriz MacColl and L. Beyer, Inorg. Chem., 12, 7 (1973).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hay, R.W., McLaren, F.M. Kinetics of base hydrolysis of [Co(tetren)dmf]3+ and [Co(tetren)-dmso]3+ (tetren=1,11-diamino-3,6,9-triazaundecane). Transition Metal Chemistry 23, 143–146 (1998). https://doi.org/10.1023/A:1006943009389
Issue Date:
DOI: https://doi.org/10.1023/A:1006943009389