Abstract
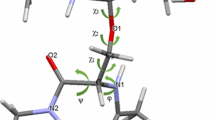
The novel glycosphingolipid, SEGLx (Gal beta 1-4(Fuc alpha 1-3)Glc beta 1-3Gal beta Cer), which was identified by us (Kawakami Y, et al. (1993) J Biochem 114: 677-83), shows a characteristic spectrum on 1H-NMR analysis, in which the anomeric proton resonances of a reducing end galactose and a glucose are split. To elucidate the structural characteristics of SEGLx, we determined its three-dimensional (3D) structure by means of computer simulation, involving such techniques as molecular mechanics (MM2), the semiempirical molecular orbital method (AM1), molecular dynamics (Amber), and computer 3D modelling. With the hypothesis that all OH group(s) of a ceramide participate in intramolecular hydrogen bonds, two kinds of stable conformers, horizontal and right-angled ones, were formed, depending on the ceramide species. The present findings suggest that the chemical species of both the long chain base and fatty acid moieties, mainly the occurrence of OH group(s), affect the chemical shifts of the anomeric proton resonances not only of the reducing terminal galactose but also the penultimate glucose through the formation of intramolecular hydrogen bonds. Computer simulation through theoretical calculation and 3D modelling was shown to be the best means of confirming the results obtained by experimental analysis.
Similar content being viewed by others
References
Karlsson KA (1989) Ann Rev Biochem 58: 309-50.
Hakomori S (1990) J Biol Chem 265: 18713-16.
Kawakami Y, Nakamura K, Kojima H, Suzuki M, Inagaki F, Suzuki A, Sonoki S, Uchida A, Murata Y, Tamai Y (1993) J Biochem 114: 677-83.
Imberty A, Mikros E, Koca J, Mollicone R, Oriol R, Perez S (1995) Glycoconjugate J 12: 331-49.
Burkert U, Allinger NL (1982) Molecular Mechanics. Washington, DC: The American Chemical Society.
Clark T (1985) A Handbook of Computational Chemistry. New York: John Wiley & Sons, Inc.
Osawa E, Goto H, Oishi T, Ohtsuka Y, Chuman T (1989) Pure Appl Chem 61: 597-600.
Dewar MJS, Zoebisch EG, Healy EF, Stewart JJP (1985) J Am Chem Soc 107: 3902-9.
Weiner SJ, Kollman PA, Case DA, Singh VS, Chio C, Alagon GS, Profeta S, Weiner P (1984) J Am Chem Soc 106: 765-84.
Weiner P, Kollman PA, Nguyen DT, Case DA (1986) J Comp Chem 7: 230-52.
Nyholm P, Pascher I, Sundell S (1990) Chem Phys Lipids 52: 1-10.
Hardy BJ, Sarko A (1993) J Comp Chem 14: 848-57.
Dowd MK, Reilly PJ, French AD (1992) J Comp Chem 13: 102-14.
Jeffery GA, Taylor R (1980) J Comp Chem 1: 99-109.
Yamada H (1997) J Syn Org Chem 55: 29-41.
Dabrowski J, Egge H, Hanfland P (1980) Chem Phys Lipids 26: 187-96.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kawakami, J., Kawakami, Y., Nakamura, K. et al. Three-dimensional structure of a glycosphingolipid having a novel carbohydrate linkage, Gal beta 1-4(Fuc alpha 1-3)Glc beta 1-3Gal beta, determined by theoretical calculations. Glycoconj J 15, 107–113 (1998). https://doi.org/10.1023/A:1006908020370
Issue Date:
DOI: https://doi.org/10.1023/A:1006908020370