Abstract
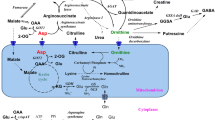
Lysine, an essential amino acid is catabolized in brain through only the pipecolic acid pathway. During the formation of pipecolic acid, α-deamination of lysine, and the formation of the α-keto acid as well as its cyclized product are pre-requisites. The enzyme mediated α-deamination of L-lysine and the formation of the α-keto acid and the cyclized product are not demonstrated so far. Both lysine and pipecolic acid are known to increase in brain under the conditions of fasting, studies were therefore undertaken to identify the enzyme responsible for the α-deamination of L-lysine in the brain tissue of mice which were fasted. The detection of the α-keto acid of L-lysine, α-keto-ε-amino caproic acid and its cyclized product, Δ 1-piperidine-2-carboxylate was facilitated by the use of L-[U-14-C]-lysine as the substrate. The quantitation of the radioactivity in reaction products was done after separation by ion exchange chromatographic methods. The formation of the α-keto acid was enzyme mediated, the α-keto acid formed was established by reaction with N-methyl benzothiazolinone hydrazone hydrochloride. The cyclized product was accounted in a fraction which matched the resolution of authentic pipecolic acid on the Dowex column, and the cyclized product was confirmed by spectrophotometry. The hitherto undemonstrated α-amino deaminating enzyme of L-lysine in brain tissue, the α-keto acid of L-lysine and its cyclized product in a mammalian system could thus be demonstrated in the present study. These findings confirm the involvement of L-lysine oxidase/L-amino acid oxidase in the formation of pipecolic acid from L-lysine.
Similar content being viewed by others
References
Meister A: Intermediary Metabolism of Amino Acids. In: Biochemistry of Amino Acids, 2nd edn. Academic Press, New York, Vol. 11 1965, pp 928–1020
Scriver CR, Rosenberg LE: Lysine. In: Amino Acid Metabolism and its Disorders. Saunders, Philadelphia, 1973, pp 250–255
Higashino K, Tsukada K, Lieberman J: Saccharopine, a product of lysine breakdown in mammalian liver. Biochem Biophys Res Commun 20: 285–290, 1965
Higashino K, Fujioka M, Akoi T, Yamamura Y: Metabolism of lysine in rat liver. Biochem Biophys Res Commun 29: 95–100, 1967
Higashino K, Fujioka M, Yamamura Y: The conversion of L-Lysine to saccheropine and α-amino adipate in mouse. Arch Biochem Biophys 142: 606–614, 1971
Hutzier J, Dancis J: Conversion of lysine to saccharopine by human tissues. Biochem Biophys Acta 158: 62–69, 1968
Chang YF: Lysine metabolism in the rat brain: The pipecolic acid forming pathway. J Neurochem 30: 347–354, 1978
Dagley S, Nicholson DE: Amino Acids in An Introduction to Metabolic Pathways. Blackwell Science Publishing, Oxford, 1970, pp 190–249
Chu SW, Hegsted DM: Adaptive response of lysine and threonine degrading enzymes in adult rats. J Nutrition 106: 1089–1096, 1976
Torchinsky Yu M: Transamination: Its discovery, biological and chemical aspects. (1937–1987) Trends Biochem Sci 12: 115–117, 1987
Broquist P: Lysine-Pipecolic Acid relationship in microbes and mammals. In: Ann. Review of Nutrition 11 1991 pp 435–448
Rodwell VW: Catabolism of Carbon Skeletons of Amino Acids. In: R.K. Murray, D.K. Graner, P.A. Mayes, V.W. Rodwell (eds). Harpers Biochemistry, 23rd edn. Prentice Hall Int. Inc., 1993, pp 303–325
Boulanger E, Bertrand J, Oesteux R: Desamination de l'orinithine et de LA lysine selectivement marquees par la L-amino acide-dehydrogenase du foie de dindou (Meleagris Calloparo L). Biochem Biophys Acta 26: 143–145, 1957
Nishio H, Sawda E, Sogabe H, Segawa T: Effect of starvation and immobilization on amino acids in mouse brain and peripheral tissues. Neurochem Int 8(2): 229–233, 1986
Weismann N, Schoenheimer R: The relative stability of L(+)-Lysine in rats studied with deuitirium and heavy nitrogen. J Biol Chem 140: 779–795, 1941
Rothstein M, Miller LL: The conaversion of L-lysine-6–14C to pipecolic acid in the rat. J Am Chem Soc 75: 4371–4372, 1953
Rothstein M, Miller LL: The conversion of L-lysine to pipecolic acid in the rat. J Biol Chem 211: 851–858, 1954
Schmidt-Glenewinkel T, Nomura Y, Glacobini E: The conversion of lysine into piperidine, cadaverine, and pipecolic acid in the brain and other organs of the mouse. Neurochem Res 2: 619–637, 1977
Giacobini E, Nomura Y, Schmidt-Glenewinkel T: Pipecolic acid: Origin, biosynthesis and metabolism in the brain. Cell Mol Biol 26: 135–145, 1980
Gatfield PD, Taller E, Hinton GG, Wallace AC, Abdelnoum GM, Haust MD: Hyperpipecolatemia: A new metabolic disorder associated with neuropathy and hepatomegaly. Can Med Assoc J 99: 1215–1233, 1968
Thomas GH, Hasiam RH, Batshaw ML, Caputa AJ, Neidengard L, Raonsom JL: Hyperpipecolic acidemia associated with hepatomegaly, mental retardation, optic nerve dysplasia and progressive neurological disease. Clin Genet 8: 376–382, 1975
Trijbels JMF, Monnens LAH, Bakkeren JAJAM, Raay Selten AHJ, Corsteaensen JMB: Biochemical studies in the cerebro-hepato-renal syndrome of Zellweger: A disturbance in the metabolism of pipecolic acid. J Inher Metab Dis 2: 39–42, 1979
Burton BK, Reed SP, Remy WT: Hyperpipecolic acidemia: Clinical and biochemical obsersvations in two male siblings. J Pediat 99: 729–734, 1981
Chalia VR, Geisinger KR, Burton BK: Pathological alteration in the brain and liver in hyperpipecolic acidemia. J Neuropath Exp Neurol 42: 627–638, 1983
Kelley RI, Moser HW: Hyperpipecolic aciduria in neonatal adrenoleukodystrophy. Am J Med Genet 19: 791–795, 1984
Kase Y, Takahama K, Hashimoto T, Kaisaku J, Okano Y, Miyata T: Electrophoretic study of pipecolic acid a biogenic imino acid, in the mammalian brain. Brain Res 193: 608–613, 1980
Nomura Y, Okuma Y, Segawa T, Schmidt-Glenewinkwel T, Giacobini E: Comparison of synaptosomal and glial uptake of pipecolic acid and GABA in rat brain. Neurochem Res 6: 391–400, 1981
Giacobini E: Imino acids of the brain. In: A. Lajtha (ed). Hand book of Neurochemistry, 2nd edn. Raven Press, Vol. 3, 1983, pp 583–605
Feigenbaum P, Chang YF: Pipecolic acid antagonizes barbiturate enhanced GABA binding to bovine brain membranes. Brain Res 372: 176–179, 1986
Meister A, Radhakrishnan AN, Buckley SD: Enzymatic synthesis of L-pipecolic acid and L-proline. J Biol Chem 229: 789–800, 1957
Murthy SN, Janardana Sarma MK: Enzymatic conversion of L-lysine to α-keto ∈-amino caproic acid. (Abstr) J Neurochem 63 (Suppl.1): S28 D, 1994
Kusakabe H, Kodama K, Kuninaka A, Yashino H, Misono H, Soda K: A new antitumor enzyme, L-lysine α-oxidase from Trichoderma viride. J Biol Chem 255: 976–981, 1980
Lajtha A: In: A Lajtha (ed). Handbook of Neurochemistry. 2nd edn. Vol IV. 1983, pp 77–110
Murthy SN, Sarma MKJ: Detection of the cyclised product of α-keto acid of L-lysine-precursor for the formation of pipecolic acid. (Abstract), Annual Meeting of Society of Biological Chemists (India) 1996
Bradford MM: A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem 72: 248–254, 1976
Soda K: Microdetermination of D-amino acids and D-amino acid oxidase activity with 3–methyl-2–benzothiazolone hydrazone hydrochloride. Anal Biochem 25: 228–235, 1968
Rodwell VW: Pipecolic acid. In: H. Tabor, C.W. Tabor (eds). Methods in Enzymology XVII B, 1971, pp 174–188
Katsoyannis PG, Schwartz GP: The synthesis of peptides by homogenous solution procedures. In: C.H.W. Hirs, S.W. Timasheff (eds). Methods in Enzymology XLVII, 1979, pp 501–578
Knott RJ, Joseph MH, Curzon G: Effects of food deprivation and immobilisation on tryptophan and other amino acids in rat brain. J Neurochem 20: 249–280, 1973
Volpe JJ, Lee G, Laster L, Robinson JC: Regional distribution of isoenzymes of D-amino acid oxidase and acetyl esterases in developing primate brain. Exp Neurol 28: 76–87, 1970
Weliner D, Lichtenberg LA: Assay of amino acid oxidase. In: H. Tabor, C.W. Tabor (eds). Methods in Enzymology XVII B, 1971, pp 593–596
Ratner S: A long view of nitrogen metabolism. In: E.E. Snell, P.D. Boyer, A. Meister, C.L. Richardson (eds). Ann Rev Biochem 46: 1977, pp 1–24
Soda K, Misono H: L-lysine α-ketoglutarate amino transferase (Achromobacter liquidum) In: H. Tabor, C.W. Tabor (eds). Methods in Enzymology XVII B, 1971, pp 222–223
Rao DR, Rodwell VW: Metabolism of pipecolic acid in a Pseudomonas species. J Biol Chem 237: 2232–2238, 1962
Basso LV, Rao DR, Rodwell VW: Metabolism of pipecolic acid in a Pseudomonas species. J Biol Chem 237: 2239–2245, 1962
Paz MA, Blumendfeld OO, Rojkind M, Henson E, Furfine C, Gallop PM: Determinantion of carbonyl compounds with N-methyl benzothiazolone hydrazone. Arch Biochem Biophys 109: 548–559, 1965
Hamilton PB: Ion exchange chromotography of amino acids. Anal Chem 35: 2055–2064, 1963
Lehninger A: Oxidative degradation of amino acids. In: Biochemistry. Worth Publushing Inc., New York, 1970, pp 433–454
Wickwire BM, Wagner C, Broquist HP: Pipecolic acid biosynthesis in Rhizoctonia leguminicola. J Biol Chem 265: 14748–14753, 1990
Fellows FCI, Carson NAJ: Enzyme studies in a patient with saccharopinuria: A defect in lysine metabolism. Pediat Res 8: 42–49, 1974
Meister A: General Biochemical and Physiological considerations. In: Biochemistry of Amino Acids, 2nd edn. Academic Press, New York. Vol 1, 1965a, pp 269–437
Hagihara H, Hayashi H, Ichihara A, Suda M: Metabolism of L-lysine by bacterial enzymes. J Biochem (Tokyo), 48: 267–276, 1960
Bixell G, Hamprecht B: Generation of ketone bodies from leucine by cultured astroglial cells. J Neurochem 65: 2450–2461, 1995
Pevzner: Multiple forms of enzymes. In: A. Lajtha (ed). Handbook of Neurochemistry, Vol. IV, 2nd edn. 1983, pp 461–484
Hill JM: Diamine oxidase (pea seedling). In: H. Tabor, C.W. Tabor (eds). Methods in Enzymology XVII B, 1971, pp 730–735
Neuberger A, Sanger F: The availability of the acetyl derivatives of lysine for growth. Biochem J 37: 515–518, 1943
Neuberger A, Sanger F: The metabolism of lysine. Biochem J 58: 119–125, 1944
Meister A: Enzymatic preparation of α-Keto acids. J Biol Chem 197: 309–317, 1952
Hernandez MF, Chang YF: In vitro synthesis of L-pipecolate from Llysine: Inconsistent with ∈-acetyl L-lysine as an obligatory intermediate. Biochem Biophys Res Commun 93: 762–769, 1980
Murthy SN: Ph.D. Thesis - Studies on the enzymatic conversion of Llysine to pipecolic acid: Identification of L-amino acid oxidase in mouse brain. Osmaania University, Hyderabad, India, 1996
Greenstsein JP, Birnbaum SM, Otey MC: Optical and enzymatic characterization of amino acids. J Biol Chem 204: 307–321, 1953
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Murthy, S., Janardanasarma, M. Identification of L-amino acid/L-lysine α-amino oxidase in mouse brain. Mol Cell Biochem 197, 13–23 (1999). https://doi.org/10.1023/A:1006906505745
Issue Date:
DOI: https://doi.org/10.1023/A:1006906505745