Abstract
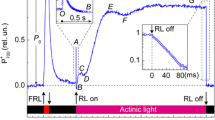
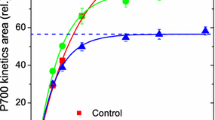
In CO2-free air, the CO2 postirradiation burst (PIB) in wheat leaves was measured with an IRGA in an open gas exchange system to ascertain its potential role in alleviating photoinhibition of photorespiratory carbon oxidation (PCO) under a CO2 deficiency. A pre-photosynthesized leaf having been transferred into CO2-free air exhibited a typical CO2 PIB following darkening which could last, with a rate substantially higher than that of dark respiration, over a long time period (at least more than 2 h) of continuously alternate irradiation (2 min)-dark (2 min)-light transitions. The rate and the time of PIB maintenance, although unaffected by the exogenous dark respiration inhibitor iodoacetic acid, were stimulated largely by increasing irradiance and O2 level, and suppressed by DCMU and N-ethyl-maleimide (NEM). They also showed a large photosynthates-loading dependence. In a darkened leaf, the irradiation-induced PIB in the CO2-free air was clearly revealed and it was characterized by an initial net uptake of respiratory CO2. The light-induced PIB was accelerated by increasing irradiance, and delayed by prolonging the period of darkening the leaves. Hence, the origin of carbon needed for a long-term CO2 evolution in the CO2-free air might not only be derived directly from the pool of intermediates in the Calvin cycle, but it might also arise indirectly from a remotely fixed reserve of photosynthates in the leaf via a PCO-mediated, yet to be further clarified, mobilization process. Such mobilization of photosynthates probably exerted an important role in coordination of photochemical reactions and carbon assimilation during photosynthesis in C3 plants under the photoinhibitory conditions.
Similar content being viewed by others
References
Beck, E., Ziegler, P.: Biosynthesis and degradation of starch in higher plants.-Annu. Rev. Plant Physiol. Plant mol. Biol. 40: 95–117, 1989.
Cardini, C.E., Leloir, L.F., Chiriboga, J.: The biosynthesis of sucrose.-J. biol. Chem. 214: 149–155, 1955.
Caspar, T., Lin, T.P., Kakefuda, G., Benbow, L., Preiss, J., Somerville, C.: Mutants of Arabidopsis with altered regulation of starch degradation.-Plant Physiol. 95: 1181–1188, 1991.
Ching, T.M., Poklemba, C.J., Metzgen, R.J.: Starch synthesis in shriveled and plump triticale seeds.-Plant Physiol. 73: 652–657, 1984.
Cornic, C., Gaudillère, J.P.: Méthodes de measures du dégagement de gaz carbonique photorespiratoire.-Physiol. vég. 19: 301–313, 1981.
Cowan, I.R.: Stomatal behavior and environment.-Adv. bot. Res. 4: 117–122, 1977.
Decker, J.P.: A rapid postillumination deceleration of respiration in green leaves.-Plant Physiol. 30: 82–84, 1955.
Decker, J.P.: Comparative responses of carbon dioxide outburst and uptake in tobacco.-Plant Physiol. 34: 100–102, 1959.
Doehlert, D.C., Ku, M.S.B., Edwards, G.E.: Dependence of the post-illumination burst of CO2 on temperature, light, CO2 and O2 concentration in wheat (Triticum aestivum).-Plant Physiol. 46: 299–306, 1979.
Edwards, G., Walker, D.A. (ed.): C3, C4: Mechanisms, and Cellular and Environmental Regulation of Photosynthesis.-Pp. 368–373. Blackwell Sci. Publ., Oxford 1983.
Fock, H., Klug, K., Canvin, D.T.: Effect of carbon dioxide and temperature on photosynthetic CO2 uptake and photorespiratory CO2 evolution in sunflower leaves. Planta 145: 219–223, 1979.
Fox, T.C., Geiger, D.R.: Effects of decreased net carbon exchange on carbohydrate metabolism in sugar beet source leaves.-Plant Physiol. 76: 763–768, 1984.
Hitz, W.D., Stewart, C.R.: Oxygen and carbon dioxide effects on the pool size of some photosynthetic and photorespiratory intermediates in soybean (Glycine max [L.] Merr).-Plant Physiol. 65: 442–446, 1980.
Knapp, A.K., Smith W.K.: Stomatal and photosynthetic responses to variable sunlight.-Physiol. Plant. 78: 160–165, 1990.
Krause, G.H.: Photoinhibition of photosynthesis. An evaluation of damaging and protective mechanisms.-Physiol. Plant. 74: 566–574, 1988.
Krause, G.H., Cornic, G.: CO2 and O2 interactions in photoinhibition.-In: Kyle, D.J., Osmond, C.B., Arntzen, C.J. (ed.): Photoinhibition. Pp. 169–196. Elsvier Science Publishers, Amsterdam-New York-Oxford 1987.
Krause, G.H., Kirk, M., Heber, U., Osmond, C.B.: O2-dependent inhibition of photosynthetic capacity in intact isolated chloroplasts and isolated cells from spinach leaves illuminated in the absence of CO2.-Planta 142: 229–233, 1978.
Levi, C., Gibbs, M.: Starch degradation in synchronously grown Chlamydomonas reinhardtii and characterization of the amylase.-Plant Physiol. 74: 459–463, 1984.
Lorimer, G.H.: The carboxylation and oxygenation of ribulose 1,5-bisphosphate: The primary events in photosynthesis and photorespiration.-Annu. Rev. Plant Physiol. 32: 349–383, 1981.
Ludwig, L.J., Canvin, D.T.: An open gas-exchange system for the simultaneous measurement of the CO2 and 14CO2 fluxes from leaves.-Can. J. Bot. 49: 1299–1313, 1971.
Marek, M.V., Kalina, J., Matoušková, M.: Response of photosynthetic carbon assimilation of Norway spruce exposed to long-term elevation of CO2 concentration.-Photosynthetica 31: 209–220, 1995.
Neuhaus, H.E., Henrichs, G., Scheibe, R.: Starch degradation in intact amyloplasts isolated from cauliflower floral buds (Brassica oleracea L.).-Planta 195: 496–504, 1995.
Ogren, W.L.: Photorespiration: pathways, regulation and modification.-Annu. Rev. Plant Physiol. 35: 415–442, 1984.
Palovský, R., Hák, R., A model of light-dark transition of CO2 exchange in the leaf (post-illumination burst of CO2). Theoretical approach.-Photosynthetica 22: 423–430, 1988.
Pärnik, T., Keerberg, O.: Decarboxylation of primary and end products of photosynthesis at different oxygen concentration.-J. exp. Bot. 46: 1439–1447, 1995.
Peterson, R.B.: Estimation of photorespiration based on the initial rate of postillumination CO2 release. I. A nonsteady state model for measurement of CO2 exchange transients.-Plant Physiol. 73: 978–982, 1983a.
Peterson, R.B.: Estimation of photorespiration based on the initial rate of postillumination CO2 release. II. Effects of O2, CO2, and temperature.-Plant Physiol. 73: 983–988, 1983b.
Powles, S.B., Osmond, C.B.: Inhibition of the capacity and efficiency of photosynthesis in bean leaflets illuminated in a CO2-free atmosphere at low oxygen: A possible role for photorespiration.-Aust. J. Plant Physiol. 5: 619–629, 1978.
Savitch, L.V., Maxwell, D.P., Huner, N.P.A.: Photosystem II excitation pressure and photosynthetic carbon metabolism in Chlorella vulgaris.-Plant Physiol. 111: 127–136, 1996.
Scorer, K.N.: Evidence for energy-dependent 14C-photoassimilate retention in isolated tobacco mesophyll cells.-Plant Physiol. 76: 753–758, 1984.
Šesták, Z. (ed.): Photosynthesis During Leaf Development.-Academia, Praha; Dr W. Junk Publ., Dordrecht-Boston-Lancaster 1985.
Sharkey, T.D.: Estimating the rate of photorespiration in leaves.-Physiol. Plant. 73: 147–152, 1988.
Sharkey, T.D., Seemann, J.R., Pearcy, R.W.: Contribution of metabolites of photosynthesis to post-illumination CO2 assimilation in response to lightflecks.-Plant Physiol. 82: 1063–1068, 1986.
Somerville, C.R., Ogren, W.L.: Photorespiration mutants of Arabidopsis thaliana deficient in serine-glyoxylate aminotransferase activity.-Proc. nat. Acad. Sci. USA 77: 2684–2687, 1980.
Stitt, M., Heldt, H.W.: Simultaneous synthesis and degradation of starch in spinach chloroplasts in the light.-Biochim. biophys Acta 638: 1–11, 1981.
Stitt, M., Steup, M.: Starch and sucrose degradation.-In: Douce, R., Day, D. (ed.): Encyclopedia of Plant Physiology. Vol. 18. Pp. 347–390. Springer-Verlag, Berlin 1985.
Stitt, M., Wirtz, W., Heldt, H.W.: Metabolite levels during induction in the chloroplast and extrachloroplast compartments of spinach protoplasts.-Biochim. biophys. Acta 593: 85–102, 1981.
Tolbert, N.E.: The oxidative photosynthetic carbon cycle.-In: Randall, D.D., Blevins, D.G., Larson, R. (ed.): Current Topics in Plant Biochemistry and Physiology. Vol. 1. Pp. 63–77. University of Missouri, Columbia 1983.
Wardlaw, I.F.: Assimilation movement in Lolium and Sorghum leaves. III. Carbon dioxide concentration effects on the metabolism and translocation of photosynthate.-Aust. J. Plant Physiol. 9: 705–713, 1982.
Wu, J., Neimanis, S., Heber, U.: Photorespiration is more effective than the Mehler reaction in protecting the photosynthetic apparatus against photoinhibition.-Bot. Acta 104: 283–291, 1991.
Yemm, E.W.: Photorespiration and photosynthesis in young leaves of barley.-In: Metzner, H. (ed.): Progress in Photosynthesis Research. Vol. 1. Pp. 474–481. Tübingen 1969.
Zelitch, I.: Investigations on photorespiration with a sensitive 14C-assay.-Plant Physiol. 43: 1829–1837, 1968.
Zelitch, I.: Measurement of photorespiratory activity and the effect of inhibitors.-In: Colowick, S.P., Kaplan, N.O. (ed.): Methods in Enzymology. Vol. 69. Pp.453–464. Academic Press, New York-London-Toronto-Sydney-San Francisco 1980.
Zelitch, I.: Biochemical and genetic regulation of photorespiration.-In: Sybesma, C. (ed.): Advances in Photosynthesis Research. Vol. III. Pp. 811–816. Martinus Nijhoff/Dr W. Junk Publ., The Hague-Boston-Lancaster 1984.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Xiong, F., Gao, Y. & Song, P. A long-lasting photorespiration in CO2-free air, measured as the postirradiation CO2 burst, indicates mobilization of storage photosynthates. Photosynthetica 35, 107–119 (1998). https://doi.org/10.1023/A:1006882101535
Issue Date:
DOI: https://doi.org/10.1023/A:1006882101535