Abstract
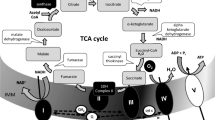
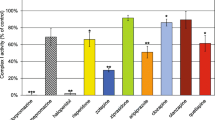
There is increasing evidence that a defect of the mitochondrial respiratory chain is implicated in the development of Parkinson disease. Decreased complex I activity of the mitochondrial respiratory chain has been reported in platelets, muscle, and brain of patients with Parkinson disease. Extrapyramidal symptoms (e.g. parkinsonism and dystonic reactions) are major limiting side effects of neuroleptics. Experimental evidence suggests that neuroleptics inhibit complex I in rat brain. There has not been a study of the effects of neuroleptics in human tissue, however. We therefore analyzed the activities of complexes I + III, complexes II + III, succinate dehydrogenase, complex IV (cytochrome c oxidase), and of citrate synthase in normal human brain cortex after the addition of haloperidol and chlorpromazine and the atypical neuroleptics risperidone, zotepine, and clozapine. Activity of complex I was progressively inhibited by all neuroleptics. Half maximal inhibition (IC50) was 0.1 mM fo r haloperidol, 0.4 mM for chlorpromazine, and 0.5 mM for risperidone and zotepine. Clozapine had no effect on enzyme activity at concentrations up to 0.5 mM, followed by a slow decline with a maximum inhibition of 70% at 10 mM. IC50 was at about 2.5 mM. Thus, the concentration of clozapine needed to cause 50% inhibition of the activity of complexes I and III was about 5 times that of zotepine and risperidone, about 6 times that of chlorpromazine, and 25 times that of haloperidol. The inhibition thus paralleled the incidence of extrapyramidal effects caused by the different neuroleptics as they are known from numerous clinical studies. Our data support the hypothesis that neuroleptic-induced extrapyramidal side effects may be due to inhibition of the mitochondrial respiratory chain. (Mol Cell Biochem 174: 255–259, 1997)
Similar content being viewed by others
References
Baron JC, Martinot JL, Cambon H et al: Striatal dopamine receptor occupancy during and following withdrawal from neuroleptic treatment: correlative evaluation by positron emission tomography and plasma prolactin levels. Psychopharmacol 99: 463–472, 1989
Schapira AHV: Evidence for mitochondrial dysfunction in Parkinson's disease – a critical appraisal. Mov Disord 9: 125–138, 1994
Parker WD, Boyson SJ, Parks JK: Abnormalities of the electron transport chain in idiopathic Parkinson's disease. Ann Neurol 26: 719–723, 1989
Shoffner JM, Watts RL, Juncos JL, Torroni A, Wallace DC: Mitochondrial oxidative phosphorylation defects in Parkinson's disease. Ann Neurol 30: 332–339, 1991
Ramsay RR, Dadgar J, Trevor A, Singer TP: Energy-driven uptake of N-methyl-4-phenylpyridine by brain mitochondria mediates the neurotoxicity of MPTP. Life Sci 39: 581–588, 1986
Subramanyam B, Rollema H, Woolf T, Castagnoli N: Identification of a potentially neurotoxic pyridinium metabolite of haloperidol in rats. Biochem Biophys Res Commun 166: 238–244, 1990
Burkhardt C, Kelly JP, Lim Y-H, Filley CM, Parker WD: Neuroleptic medications inhibit complex I of the electron transport chain. Ann Neurol 33: 512–517, 1993
Jackson Lewis V, Przedborski S, Jiang H, Fahn S: Effect of neuroleptics on the mitochondrial electron transport chain [abstract]. Soc Neurosci Abstr 18: 1103, 1992
Nicklas WJ, Vyas I, Heikkila RE: Inhibition of NADH-linked oxidation in brain mitochondria by MPP+, a metabolite of the neurotoxin MPTP. Life Sci 36: 2503–2508, 1985
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ: Protein measurement with the folin reagent. J Biol Chem 193: 265–275, 1951
Casey DE: Clozapine: neuroleptic-induced EPS and tardive dyskinesia. Psychopharmacol 99: S47–S53, 1989
Matsuda LA, Schmidt CJ, Hanson GR, Gibb JW: Effect of 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine (MPTP) on striatal tyrosine hydroxylase and tryptophan hydroxylase in rat. Neuropharmacology 25: 249–255, 1986
Wase AW, Christensen J, Polley E: The accumulation of S35-chlorpromazine in brain. Arch Neurol Psychiat 75: 54–56, 1995
Sjöstrand SE, Cassano GB, Hansson E: The distribution of 35S-chlorpromazine in mice studied by whole body autoradiography. Arch Int Pharmacodyn 156: 34–46, 1965
Lindquist NG: Accumulation in vitro of 35S-chlorpromazine in the neuromelanin of human substantia nigra and locus coeruleus. Arch Int Pharmacodyn 200: 190–195, 1972
Sunderland T, Cohen BM: Blood to brain distribution of neuroleptics. Psychiatr Res 20: 299–305, 1987
Noda K, Suzuki A, Okui M, Noguchi H, Nishiura M, Nishiura N: Pharmacokinetics and metabolism of 2-chloro-11-(2-dimethylaminoethoxy)-dibenzothiepine (zotepine) in rat, mouse, dog and man. Drug Res 29: 1595–1600, 1979
Burt DR, Creese I, Snyder SH: Antischizophrenic drugs: chronic treatment elevates dopamine binding in brain. Science 196: 326–328, 1977
Costall B, Fortune DH, Naylor RJ, Marsden CD, Pycock C: Serotoninergic involvement with neuroleptic catalepsy. Neuropharmacology 14: 859–868, 1975
Przedborski S, Jackson-Lewis V, Muthane U et al.: Chronic levodopa administration alters cerebral mitochondrial respiratory chain activity. Ann Neurol 34: 715–723, 1993
Nielsen EB, Lyon M: Evidence for cell loss in corpus striatum after long-term treatment with a neuroleptic drug (flupenthixol) in rats. Psychopharmacology 59: 85–89, 1978
Fibiger HC, Lloyd KG: Is the dopamine hypothesis of tardive dyskinesia completely wrong? Trends Neuro Sci 9: 259–260, 1986
Linnane AW, Marzuki S, Ozawa T, Tanaka M: Mitochondrial DNA mutations as an important contributor to ageing and degenerative diseases. Lancet 3: 642–645, 1989
Wallace DC: Mitochondrial genetics: A paradigm for ageing and degenerative diseases? Science 256: 628–632, 1992
Corral-Debrinski M, Horton T, Lott MT, Shoffner JM, Beal MF, Wallace DC: Mitochondrial DNA deletions in human brain: regional variability and increase with advanced age. 13 Nature Genet 2: 324–329, 1992
Soong NW, Hinton DR, Cortopassi G, Arnheim N: Mosaicism for a specific somatic mitochondrial DNA mutation in adult human brain. Nature Genet 2: 318–323, 1992
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Maurer, I., Möller, HJ. Inhibition of complex I by neuroleptics in normal human brain cortex parallels the extrapyramidal toxicity of neuroleptics. Mol Cell Biochem 174, 255–259 (1997). https://doi.org/10.1023/A:1006872911332
Issue Date:
DOI: https://doi.org/10.1023/A:1006872911332