Abstract
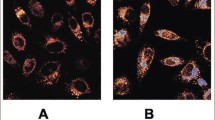
Progressive glomerulosclerosis is a major complication in patients with familial lecithin:cholesterol acyltransferase (LCAT) deficiency. The lack of LCAT activity results in the accumulation of an abnormal lipoprotein, lipoprotein-X (Lp-X), in the plasma of these patients. Lipoprotein-X contains high levels of unesterified cholesterol and phosphatidylcholine. Lp-X may play a role in the accumulation of lipids in the kidney, which in turn may lead to glomerulosclerosis. The objective of this study is to examine the uptake and metabolism of Lp-X by rat mesangial cells. Our results suggest that Lp-X is taken up by mesangial cells and that the lipids in Lp-X are metabolized. Lysosomes containing unesterified cholesterol and phosphatidylcholine, in a molar ratio similar to Lp-X, were synthesized to investigate the roles individual apolipoproteins (apo CI, II, III and E) play in the uptake of Lp-X. Both apo CI and CIII inhibited its uptake while apo CII (1.5 fold) and E (4 fold) stimulated t he uptake of Lp-X. Very low density lipoprotein (VLDL) and low density lipoprotein (LDL) inhibited Lp-X uptake by mesangial cells. However, at higher concentrations of high density lipoprotein (HDL), the uptake of Lp-X was stimulated. Proteoglycans have an important role in regulating the uptake of Lp-X, while cytoskeleton-dependent phagocytosis and the scavenger receptor do not appear to be involved. (Mol Cell Biochem 175: 187–194, 1997)
Similar content being viewed by others
References
Glomset JA, Assmann G, Gjone E, Norum KR: Lecithin:cholesterol acyltransferase deficiency and fish eye disease. In: CR Scriver, AL Beaudet, WS Sly, D Valle (eds). The Metabolic Basis of Inherited Disease. McGraw-Hill, Inc., New York, 1995, pp 1933–1951
Norum KR, Gjone E: Familial plasma lecithin:cholesterol acyltransferase deficiency. Biochemical study of a new inborn error of metabolism. Scand J Clin Lab Invest 20: 231–243, 1967
McLean JW: Molecular defects in the lecithin:cholesterol acyltransferase gene. In: NE Miller, AR Tall (eds). High Density Lipoproteins and Atherosclerosis III. Elsevier Science Publishers B.V. Amsterdam, 1992, pp 59–65
Klein H-G, Lohse P, Duverger N, Albers JJ, Rader DJ, Zech LA, Santamarina-Fojo S, Brewer HB Jr: Two different allelic mutations in the lecithin:cholesterol acyltransferase (LCAT) gene resulting in classic LCAT deficiency: LCAT(tyr83-stop) and LCAT(tyr156-asn). J Lipid Res 34: 49–58, 1993
Frohlich J, McLeod R, Pritchard PH, Fesmire J, McConathy W: Plasma lipoprotein abnormalities in heterozygotes for familial lecithin: cholesterol acyltransferase deficiency. Metabolism 37: 3–8, 1988
Hoving T, Gjone E: Familial plasma lecithin:cholesterol acyltransferase (LCAT) deficiency: ultrastructural aspects of a new syndrome with particular reference to lesions in the kidneys and the spleen. Acta Pathol Microbiol Scand Section A 81: 681–697, 1973
Gjone E, Blomhoff J, Skarbovik AJ: Possible association between an abnormal low density lipoprotein and nephropathy in lecithin: cholesterol acyltransferase deficiency. Clin Chim Acta 54: 11–18, 1974
Stokke KT, Bjerve KS, Blomhoff JP, Oystese B, Flatmark A, Norum KR, Gjone E: Familial lecithin:cholesterol acyltransferase deficiency: studies on lipid composition and morphology of tissues. J Clin Lab Invest 33: 93–100, 1974
Flatmark AL, Hovig T, Myhre E, Gjone E: Renal transplantation in patients with familial lecithin:cholesterol-acyltransferase deficiency. Trans Proc IX: 1665–1671, 1977
Myhre E, Gjone E, Flatmark A, Hovig T: Renal failure in familial lecithin-cholesterol acyltransferase deficiency. Nephron 18: 239–248, 1977
Magil A, Chase W, Frohlich J: Unusual renal biopsy findings in a patient with familial lecithin:cholesterol acyltransferase deficiency. Hum Pathol 13: 283–285, 1982
Ohta Y, Yamamoto S, Ysuchida H, Murano S, Saitoh Y, Yohjo S, Okada M: Nephropathy of familial lecithin-cholesterol acyltransferase deficiency: Report of a case. Am J Kidney Diseases VII: 41–46, 1986
Weber P, Owen JS, Desai K, Clemens MR: Hereditary lecithincholesterol acyltransferase deficiency: Case report of a German patient. Am J Clin Pathol 88: 510–516, 1987
Horina JH, Wirnsberger G, Horn S, Roob JM, Ratschek M, Holzer H, Pogglitsch H, Krejs GJ: Long-term follow-up of a patient with lecithin cholesterol acyltransferase deficiency syndrome after kidney transplantation. Transplantation 56: 322–236, 1993
Churg J, Bernstein J, Glassock RJ: Renal Disease: Classification and Atlas of Glomerular Diseases. Igaku-Shoin, New York, 1995, pp 27–41
Kashgarian M: Mesangium and glomerular disease. Lab Invest 52: 569–571, 1995
Striker GE, Striker LJ: Biology of Disease: Glomerular cell culture. Lab Invest 53: 122–131, 1985
Wheeler D, Chana RS: Interactions between lipoproteins, glomerular cells and matrix. Miner Electrolyte Metab 19: 149–164, 1993
Diamond JR, Karnovsky MJ: Focal and segmental glomerulosclerosis: Analogies to atherosclerosis. Kidney Int 33: 917–924, 1988
Keane WF, Kasiske BL, O'Donnell MP: Lipids and progressive glomerulosclerosis. A model analogous to atherosclerosis. Am J Nephrol 8: 261–271, 1988
Seidel D, Gjone E, Blomhoff JP, Geisen HP: Plasma lipoproteins in patients with familial plasma lecithin:cholesterol acyltransferase (LCAT) deficiency – studies on the apolipoprotein composition of isolated fractions with identification of Lp-X. Horm Metab Res Suppl 4: 6–11, 1974
Sabesin SM: Cholestatic lipoproteins-their pathogenesis and significance. Gastroenterology 83: 704–709, 1982
Miller JP: Dyslipoproteinaemia of liver disease. Bailliere's Clin Endocrinology Met 4: 807–833, 1990
O K, Frohlich J: Role of lecithin:cholesterol acyltransferase and apolipoprotein A-l in cholesterol esterification in lipoprotein-X in vitro. J Lipid Res 36: 2344–2354, 1995
O K, Ly M, Fang DZ, Frohlich J, Choy PC: Effect of lipoprotein-X on lipid metabolism in rat kidney. Mol Cell Biochem (in press), 1997
Harper PA, Robinson JM, Richard L, Hoover TC, Wright, TC, Karnovsky MJ: Improved methods for culturing rat glomerular cells. Kidney Int 26: 875–880, 1984
Wengeler H, Seidel D: Does lipoprotein-X (Lp-X) act as a substrate for the lecithin:cholesterol acyltransferase (LCAT)? Clin Chim Acta 45: 429–432, 1973
Ritland S, Gjone E: Quantitative studies of lipoprotein-X in familial lecithin:cholesterol acyltransferase deficiency and during cholesterol esterification. Clin Chim Acta 59: 109–119, 1975
McLeod R, Lacko AD, Pritchard PH, Frohlich J: Purification of biological active apolipoproteins by chromatofocussing. J Chromatogr 381: 271–283, 1986
O K, Hill JS, Wang X, Pritchard PH: Recombinant lecithin:cholesterol acyltransferase containing a Thr123-IIe mutation esterifies cholesterol in low density lipoprotein but not in high density lipoprotein. J Lipid Res 34: 81–88, 1993
Dobiasova M, Schutzova M: Cold labelled substrate and estimation of cholesterol esterification rate in lecithin: cholesterol acyltransferase radioassay. Physiol Bohemoslov 35: 319–327, 1986
Wheeler DC, Persaud JW, Fernando R, Sweny P, Varghese Z, Moorhead JF: Effects of low-density lipoproteins on mesangial cell growth and viability. Nephr Dial Trans 5: 185–191, 1990
Gupta S, Rifici V, Crowley S, Brownlee M, Shan Z, Schlondorff D: Interactions of LDL and modified LDL with mesangial cells and matrix. Kidney Int 41: 1161–1169, 1992
Bally MB, Nayar R, Masin D, Hope MJ, Cullis PR, Mayer LD: Liposomes with entrapped doxorubicin exhibit extended blood residence times. Biochim Biophys Acta 1023: 133–139, 1990
Mayer LD, Hope MJ, Cullis PR: Vesicles of variable sizes produced by a rapid extrusion procedure. Biochim Biophys Acta 858: 161–168, 1986
Hurt-Camejo E, Bondjers G, Camejo G: Interaction of LDL with human arterial proteoglycans stimulates its uptake by human monocyte derived macrophages. J Lipid Res 31: 443–454, 1990
Vijayagopal P, Srinivasan SR, Radhakrishnamurthy B, Berenson GS: Lipoprotein-proteoglycan complexes from atherosclerotic lesions promote cholesteryl ester accumulation in human monocytes/macrophages. Arteriosclerosis Thromb 12: 237–249, 1992
Ismail NAE, Alavi MZ, Moore S: Lipoprotein-proteoglycan complexes from injured rabbit aortas accelerate lipoprotein uptake by arterial smooth muscle cells. Atherosclerosis 105: 79–87, 1994
Ting BHP, Lee AS, Hochmuth RM: Effect of cytochalasin D on the mechanical properties and morphology of human neutrophils. Ann Biomed Eng 23: 666–671, 1995
Schlondorff D: Cellular mechanisms of lipid injury in the glomerulus. Am J Kidney Dis 22: 72–82, 1993
Nishikawa T, Kobori S, Takeda H, Higashi T, Sato Y, Sasahara T, Yano T, Kasho M, Anami Y, Shichiri M: Beta-migrating very low density lipoproteins induce foam cell formation in mouse mesangial cells. Atherosclerosis 114: 123–132, 1995
Ito H, Chimata M, Oka M, Suzuki Y, Hamada N, Fujimaki M, Oda K, Fujisawa K, Teramoto T, Nakamura K, Nagase M, Naito C: Immunohistochemical and biochemical detection of low density lipoprotein receptors in cultured rat mesangial cells. Nephron 69: 305–310, 1995
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lynn, E.G., Choy, P.C., Magil, A. et al. Uptake and metabolism of lipoprotein-X in mesangial cells. Mol Cell Biochem 175, 187–194 (1997). https://doi.org/10.1023/A:1006865420286
Issue Date:
DOI: https://doi.org/10.1023/A:1006865420286