Abstract
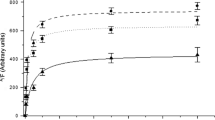
Myocardial Na+,K+-ATPase was studied in patients with aortic valve disease, and myocardial Na+,K+- and Ca2+-ATPase were assessed in spontaneously hypertensive rats (SHR) and hereditary cardiomyopathic hamsters using methods ensuring high enzyme recovery. Na+,K+-ATPase was quantified by [3H]ouabain binding to intact myocardial biopsies from patients with aortic valve disease. Aortic stenosis, regurgitation and a combination hereof were compared with normal human heart and were associated with reductions of left ventricular [3H]ouabain binding site concentration (pmol/g wet weight) of 56, 46 and 60%, respectively (p < 0.01). Na+,K+ and Ca2+-ATPases were quantified by K+- and Ca2+-dependent p-nitrophenyl phosphatase (pNPPase) activity determinations in crude myocardial homogenates from SHR and hereditary cardiomyopathic hamsters. When SHR were compared to age-matched Wistar Kyoto (WKY) rats an increase in heart-body weight ratio of 75% (p < 0.001) was associated with reductions of K+- and Ca2+-dependent pNPPase activities (μmol/min/g wet weight) of 42 (p < 0.01) and 27% (p < 0.05), respectively. When hereditary cardiomyopathic hamsters were compared to age-matched Syrian hamsters an increase in heart-body weight ratio of 69% (p < 0.001) was found to be associated with reductions in K+- and Ca2+-dependent pNPPase activities of 50 (p < 0.001) and 26% (p = 0.05), respectively. The reductions in Na+,K+- and Ca2+-ATPases were selective in relation to overall protein content and were not merely the outcome of increased myocardial mass relative to Na+,K+- and Ca2+-pumps. In conclusion, myocardial hypertrophy is in patients associated with reduced Na+,K+-ATPase concentration and in rodents with reduced Na+,K+- and Ca2+-ATPase concentrations. This may be of importance for development of heart f in hypertrophic heart disease.
Similar content being viewed by others
References
Chambers J: Left ventricular hypertrophy. Br Med J 311: 273, 1995
Skou JC: Enzymatic basis for active transport of Na+ and K+ across cell membrane. Physiol Rev 45: 596–617, 1965
Philipson KD: The Cardiac Na+,Ca2+-exchanger In: GA Langer (ed). Calcium and the Heart. Raven Press, New York, 1990, pp 85–108
Carafoli E: Intracellular calcium homeostasis. Ann Rev Physiol 56: 395–433, 1987
Hansen O, Clausen T: Studies on sarcolemma components may be misleading due to inadequat recovery. FEBS 384: 203, 1996
Nørgaard A, Bagger JP, Bjerregaard P, Baandrup U, Kjeldsen K, Thomsen PE: Relation of left ventricular function and Na+,K+-pump concentration in suspected idiopathic dilated cardiomyopathy. Am J Cardiol 61: 1312–1315, 1988
Schmidt TA, Svendsen JH, Haunsø S, Kjeldsen K: Quantification of the total Na+,K+-ATPase concentration in atria and ventricles from mammalian species by measuring [ 3 H]ouabain binding to intact sam-ples. Stability to short term ischemia reperfusion. Basic Res Cardiol 85: 411–427, 1990
Larsen JS, Kjeldsen K: Quantification in crude homogenates of rat myocardial Na+,K+-and Ca2+-ATPase by K+-and Ca2+-dependent pNPPase. Age dependent changes. Basic Res Cardiol 90: 323–331, 1995
Panagia V, Singh JN, Anand Srivastava MB, Pierce GN, Jasmin G, Dhalla NS: Sarcolemmal alterations during the development of genetically determined cardiomyopathy. Cardiovasc Res 18: 567–572, 1984
Sulakhe PV, Dhalla NS: Alterations in the activity of cardiac Na+,K+-stimulated ATPase in congestive heart failure. Exp Mol Pathol 18: 100–111, 1973.
Nørgaard A, Baandrup U, Larsen JS, Kjeldsen K: Heart Na+, K+-ATPase activity in cardiomyopathic hamsters as estimated from K+-dependent 3-O-MFPase activity in crude homogenates. J Mol Cell Cardiol 19: 589–594, 1987
Yazaki Y, Fujii J: Depressed Na+,K+-ATPase activity in the failing rabbit heart. Jpn Heart J 13: 73–83, 1972
Prasad K, Khatter JC, Bharadwaj B: Intra-and extracellular electrolytes and sarcolemmal ATPase in the failing heart due to pressure overload in dogs. Cardiovasc Res 13: 95–104, 1979
Kjeldsen K, Bjerregaard P, Richter EA, Thomsen PE, Nørgaard, A: Na+,K+-ATPase concentration in rodent and human heart and skeletal muscle: apparent relation to muscle performance. Cardiovasc Res 22: 95–100, 1988
Chen CC, Lin Shiau SY: Decreased Na+,K+-ATPase activity and [3H]ouabain binding sites in various tissues of spontaneously hypertensive rats. Eur J Pharmacol 122: 311–319, 1986
Sharma RV, Butters CA, Bhalla RC: Alterations in the plasma membrane properties of the myocardium of spontaneously hypertensive rats. Hypertension 8: 583–591,1986
Aalkjaer C, Kjeldsen K, Nørgaard A, Clausen T, Mulvany MJ: Ouabain binding and Na+ content in resistance vessels and skeletal muscles of spontaneously hypertensive rats and K+-depleted rats. Hypertension 7: 277–286, 1985
Charlemagne D, Orlowski J, Oliviero P, Rannou F, Beuve CS, Swynghedauw B, Lane LK: Alteration of Na+,K+-ATPase subunit mRNA and protein levels in hypertrophied rat heart. J Biol Chem 269: 1541–1547, 1994
Magyar CE, Wang J, Azuma KK, McDonough AA: Reciprocal regulation of cardiac Na+, K+-ATPase and Na+/Ca2+-exchanger: hypertension, thyroid hormone, development. Am J Physiol 269: C675–C682, 1995
Book CS, Moore RL, Semanchik A, Ng Y: Cardiac hypertrophy alters expression of Na+,K+-ATPase subunit isoforms at mRNA and protein levels in rat myocardium. J Mol Cell Cardiol 26: 591–600, 1994
Sweadner KJ, Herrera VLM, Amato S, Moellmann A, Gibbons DK, Repke KRH: Immunologic identification of Na+,K+-ATPase isoforms in myocardium. Circ Res 74: 669–678, 1994
Morgan JP: Abnormal intracellular modulation of calcium as a major cause of cardiac contractile dysfunction. N Engl J Med 325: 625–632, 1991
de la Bastie D, Levitsky D, Rappaport L, Mercadier JJ, Marotte F, Wisnewsky C, Brovkovich V, Schwartz K, Lompre AM: Function of the sarcoplasmic reticulum and expression of its Ca2+-ATPase gene in pressure overload-induced cardiac hypertrophy in the rat. Circ Res 66: 554–64, 1990
O'Brien PJ, Shen H, Weiler J, Mirsalimi M, Julian R: Myocardial Ca 2+-sequestration failure and compensatory increase in Ca 2+-ATPase with congestive cardiomyopathy: kinetic-characterization by a ho-mogenate microassay using real-time ratiometric-indo-1-spectro-fluorometry. Mol Cell Biochem 102: 1–12, 1991
Nørgaard A, Kjeldsen, K: Human myocardial Na+,K+-pumps in relation to heart disease. J Appl Cardiol 4: 239–245, 1989
Nørgaard A, Bjerregaard P, Baandrup U, Kjeldsen K, Reske Nielsen E, Thomsen PE: The concentration of the Na+,K+-pump in skeletal and heart muscle in congestive heart failure. Int J Cardiol 26: 185–190, 1990
Schmidt TA, Holm-Nielsen P, Kjeldsen K: No upregulation of digitalis glycoside receptor (Na+,K+-ATPase) concentration in human heart left ventricle samples obtained at necropsy after long term digitalisation. Cardiovasc Res 25: 684–691, 1991
Schmidt TA, Allen PD, Colucci WS, Marsh JD, Kjeldsen K: No adaption to digitalization as evaluated by digitalis receptor (Na+,K+-ATPase) quantification in explanted hearts from donors without heart disease and from digitalized recippients with endstage heart failure. Am J Cardiol 71: 110–114, 1993
Schwinger RH, Böhm M, Erdmann E: Effectiveness of cardiac glycosides in human myocardium with and without ‘downregulated’ beta-adrenoceptors. J Cardiovasc Pharmacol 15: 692–697, 1990
Schwinger RH, Böhm M, La Rosee K, Schmidt U, Schulz C, Erdmann E: Na+-channel activators increase cardiac glycoside sensitivity in failing human myocardium. J Cardiovasc Pharmacol 19: 554–561, 1992
Shamraj Ol, Grupp IL, Grupp G, Melvin D, Gradoux N, Kremers W, Lingrel JB, De Pover, A: Characterisation of Na+,K+-ATPase, its isoforms, and the inotropic response to ouabain in isolated failing human hearts. Cardiovas Res 27: 2229–2237, 1993
Ellingsen O, Holthe MR, Svindland A, Aksnes G, Sejersted OM, llebekk A: Na+,K+-pump concentration in hypertrophied human hearts. Eur Heart J 15: 1184–1190, 1994
Hasenfuss G, Reinecke H, Studer R, Meyer M, Pieske B, Holtz J, Holubarsch C, Posival H, Just H, Drexler H: Relation between myo-cardial function and expression of sarcoplasmic reticulum Ca2+-ATPase in failing and nonfailing human myocardium. Circ Res 75: 434–442, 1994
Studer R, Reinecke H, Bilger J, Eschenhagen T, Bohm M, Hasenfuss G, Just H, Holtz J, Drexler H: Gene expression of the cardiac Na+, Ca 2+-exchanger in end-stage human heart failure. Circ Res 75: 443–453, 1994
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ: Protein measurement with the folin phenol reagent. J Biol Chem 193: 265–275, 1951
Nørgaard A, Kjeldsen K, Hansen O, Clausen T, Larsen CG, Larsen FG: Quantification of the [3H]ouabain binding site concentration in human myocardium: a postmortem study. Cardiovasc Res 20: 428–435, 1986
Skou JC, Esmann M: Preparation of membrane-bound and of solubilized Na+,K+-ATPase from rectal glands of Squalus acanthias. The effect of preparative procedures on purity, specific and molar activity. Biochim Biophys Acta 567: 436–444, 1979
Luccchesi PA, Sweadner KJ: Postnatal changes in Na+,K+-ATPase isoform expression in rat cardiac ventricle. J Biol Chem 266: 9327–9331, 1991
Dauncey MJ, Burton KA: [ 3 H]ouabain binding sites in porcine skeletal muscle as influenced by environmental temperature and energy intake. Pflugers Arch 414: 317–323, 1989
Nørgaard A, Kjeldsen K, Hansen O: K+-dependent 3-O-methylfluorescein phosphatase activity in crude homogenate of rodent heart ventricle: effect of K+-depletion and changes in thyroid status. Eur J Pharmacol 113: 373–382, 1985
O'Gorman DJ, Sheridan DJ: Abnormalities of the coronary circulation associated with left ventricular hypertrophy. Clin Sci 81: 703–713, 1991
Tsuruya Y, Ikeda U, Yamamoto K, Seino Y, Kanbe T, Shimada K: Decreased Na+, K+-ATPase gene expression in cardiomyopathic hamster hearts. Life Sci 54: 71–77, 1994
Jasmin G, Proschek L: Hereditary polymyopathy and cardiomyopathy in the Syrian hamster. I. Progression of heart and skeletal muscle lesions in the OM-X7.1 line. Muscle Nerve 5: 20–25, 1982
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Stenfatt Larsen, J., Andersen Schmidt, T., surname, H. et al. Reduced concentration of myocardial Na+,K+-ATPase in human aortic valve disease as well as of Na+,K+- and Ca2+-ATPase in rodents with hypertrophy. Mol Cell Biochem 169, 85–93 (1997). https://doi.org/10.1023/A:1006851411650
Issue Date:
DOI: https://doi.org/10.1023/A:1006851411650