Abstract
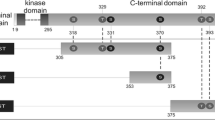
We have characterized several subdomains of the β subunit of protein kinase CK2. The N-terminal half of the protein exhibits a pseudo-substrate segment in tandem with a polyamine binding domain responsible for the activation of the kinase by these polybasic compounds. Study of the chemical features of this polyamine binding site showed that polyamine analogs exhibiting the highest affinity for CK2 are the best CK2 activators. Mutational analysis disclosed that glutamic residues lying in the polyacidic region of the CK2β subunit are involved in the interaction with polyamine molecules and allowed the delineation of an autonomous binding domain. Furthermore, this regulatory domain was shown to mediate the association of CK2 with plasma membrane.
The C-terminal domain of the CK2β subunit plays a role in the oligomerization of the kinase since it was observed that a truncated form of this subunit lacking its 33-last amino acids was incompetent for the assembly of polymeric forms of CK2. Altogether, our results support the notion that the β subunit of CK2 is a modular protein made by the association of interdependent domains that are involved in its multiple functions.
Similar content being viewed by others
References
Padmanabha R, Glover CVC: Casein kinase 2 of yeast contains two distinct α polypeptides and an unusually large β subunit. J Biol Chem 262: 1829–1835, 1987
Cochet C, Chambaz EM: Oligomeric structure and catalytic activity of G-type casein kinase. J Biol Chem 258: 1403–1406, 1983
Traugh JA, Lin WJ, Takada-Axelrod F, Tuazon PT: In: Y. Nishizuka (ed). The Biology and Medicine of Signal Transduction. Raven Press, New York, 1990, pp 224–229
Filhol O, Cochet C, Wedegaertner P, Gill GN, Chambaz EM: Co-expression of both α and β subunits is required for assembly of regulated casein kinase II. Biochemistry 30: 11133–11140, 1991
Meggio F, Boldyreff B, Marin O, Merchiori F, Perich JW, Issinger OG, Pinna LA: The effect of polylysine on casein kinase 2 activity is influenced by both the structure of the protein/peptide substrates and the subunit composition of the enzyme. Eur J Biochem 205: 939–945, 1992
Bidwai AP, Reed JC, Glover WC: Phosphorylation of calmodulin by the catalytic subunit of casein kinase 2 is inhibited by the regulatory subunit. Arch Biochem Biophys 300: 265–270, 1993
Lin WJ, Tuazon PT, Traugh JA: Characterization of the catalytic subunit of casein kinase 2 expressed in Escherichia coli and regulation of activity. J Biol Chem 266: 5664–5669, 1991
Filhol O, Cochet C, Delagoutte T, Chambaz EM: Polyamine binding activity of casein kinase 2. Biochem Biophys Res Commun 180: 945–952, 1991
Valero E, De Bonis S, Filhol O, Wade RH, Langowski J, Chambaz EM, Cochet C: Quaternary structure of casein kinase 2. Characterization of multiple oligomeric states and relation with its catalytic activity. J Biol Chem 270: 8345–8352, 1995
Leroy D, Schmidt N, Behr JP, Filhol O, Pares S, Garin J, Bourgarit JJ, Chambaz EM, Cochet C: Direct identification of a polyamine binding domain on the regulatory subunit of protein kinase CK2 by photoaffinity labeling. J Biol Chem 270: 17400–17406, 1995
Cochet C, Job D, Pirollet F, Chambaz EM: Cyclic nucleotide independent casein kinase (G-type) in bovine adrenal cortex. Purification and properties of two molecular forms. Biochim Biophys Acta 658: 191–201, 1981
Leroy D, Hériché JK, Filhol O, Chambaz EM, Cochet C: Binding of polyamines to an autonomous domain of the regulatory subunit of protein kinase CK2 induces a conformation change in the holoenzyme. J Biol Chem 272: 20820–20827, 1997
Leroy D, Filhol O, Delcros JG, Pares S, Chambaz EM, Cochet C: Chemical features of the protein kinase CK2 polyamine binding site. Biochemistry 36: 1242–1250, 1997
Ray TK: Hormone action at the membrane level. Properties of adenyl cyclase in isolated plasma membranes of rat liver. Biochim Biophys Acta 296: 1–9, 1970
Cochet C, Chambaz EM: Polyamine-mediated protein phosphorylation: A possible target for intracellular polyamine action. Mol Cell Endocrinol 30: 247–266, 1983
Boldyreff B, Meggio F, Pinna LA, Issinger OG: Efficient autophosphorylation and phosphorylation of the β subunit by casein kinase 2 require the integrity of an acidic cluster 50 residues dowstream from the phosphoacceptor site. J Biol Chem 269: 4827–4831, 1994
Boldyreff B, Meggio F, Pinna LA, Issinger OG: Reconstitution of normal and hyperactivited forms of casein kinase 2 by variably mutated β subunits. Biochemistry 32: 12672–12677, 1993
House C, Kemp BE: Protein kinase C contains a pseudo-substrate prototope in its regulatory domain. Science 238: 1726–1728, 1987
Kemp BE, Pearson RB, Guerriero V Jr, Bagchi IC, Means AR: The calmodulin binding domain of chicken smooth muscle myosin light chain kinase contains a pseudo substrate sequence. J Biol Chem 262: 2542–2548, 1987
Payne ME, Fong YL, Ono T, Colbran RJ, Kemp BE, Soderking TR, Means AR: Calcium/calmodulin-dependent protein kinase 2. J Biol Chem 263: 7190–7195, 1988
Davietov B, Sontag JM, Hata Y, Pentrenko AG, Fykse EM, Jahn R, Slidhof TC: Phosphorylation of synapto-gamin 1 by casein kinase 2. J Biol Chem 268: 6816–6822, 1993
Guerra B, Götz C, Wagner P, Montenarh M, Issinger OG: The carboxy terminus of p53 mimics the polylysine effect of protein kinase CK2. Oncogene 14: 2683–2688, 1997
Job D, Cochet C, Dhien A, Chambaz EM: A rapid method for screening inhibitor effects: Determination of I50 and its standard deviation. Anal Biochem 84: 68–77, 1978
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Leroy, D., Filhol, O., Quintaine, N. et al. Dissecting subdomains involved in multiple functions of the CK2β subunit. Mol Cell Biochem 191, 43–50 (1999). https://doi.org/10.1023/A:1006832312169
Issue Date:
DOI: https://doi.org/10.1023/A:1006832312169