Abstract
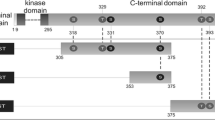
Human recombinant CK2 subunits were incubated for different times with the two main cytosolic proteases m-calpain and 20 S proteasome. Both, m-calpain in a calcium dependent manner and the 20 S proteasome, were able to degrade CK2 subunits in vitro. In both cases, CK2α′ was more resistant to these proteases than CK2α. When these proteases were assayed on the reconstituted (α2β2 holoenzyme, a 37 kDa α-band, analogous to that observed in AML extracts, was generated which was resistant to further degradation. No degradation was observed when the 26 S proteasome was assayed on free subunits. Studies with CK2α deletion mutants showed that m-calpain and the 20 S proteasome acted on the C-terminus end of CK2α. These results pointed to cytosolic proteases as agents involved in the control of the amount of free CK2 subunits within the cell, which becomes evident when CK2 is overexpressed as in AML cells.
Similar content being viewed by others
References
Pinna LA: Casein kinase 2: An ‘eminence grise’ in cellular regulation? Biochim Biophys Acta 1054: 267–284, 1990
Tuazon PT, Thaugh JA: Casein kinase I and II-multipotential serine protein kinases: Structure, function, and regulation. Adv Second Messenger Phosphoprot Res 23: 123–163, 1991
Issinger O-G: Casein kinases: Pleiotropic mediators of cellular regulation. Pharmac Ther 59: 1–30, 1993
Grankowski N, Boldyreff B, Issinger O-G: Isolation and characterization of recombinant human casein kinase II subunits α and β from bacteria. Eur J Biochem 198: 25–30, 1991
Meggio F, Boldyreff B, Marin O, Pinna LA, Issinger O-G: Role of the β subunit of casein kinase-2 on the stability and specificity of the recombinant reconstituted holoenzyme. Eur J Biochem 204: 293–297, 1992
Lüscher B, Litchfield DW: Biosynthesis of casein kinase II in lymphoid cell lines. Eur J Biochem 220: 521–526, 1994
Meisner H, Czech M: Phosphorylation of transcriptional factors and cell-cycle-dependent proteins by casein kinase II. Recent Adv Cell Mol Biol 4: 17–22, 1992
Litchfield DW, Dobrowolska G, Krebs EG: Regulation of casein kinase II by growth factors: A reevaluation. Cell Mol Biol Res 40: 373–381, 1994
Pepperkok R, Lorenz P, Jakobi R, Ansorge W, Pyerin W: Cell growth stimulation by EGF: Inhibition through antisense-oligodeoxynucleotides demonstrates important role of casein kinase II. Exp Cell Res 197: 245–253, 1991
Lorenz P, Pepperkok R, Ansorge W, Pyerin W: Cell biological studies with monoclonal and polyclonal antibodies against human casein kinase II subunit demonstrate participation of the kinase in mitogenic signaling. J Biol Chem 260: 2733–2739, 1993
Pepperkok R, Lorenz P, Ansorge W, Pyerin W: Casein kinase II is required for transition of Go/G1, early G1, and Gl/S phases of the cell cycle. J Biol Chem 269: 6986–6991, 1994
Prowald K, Fischer H, Issinger O-G: Enhanced casein kinase II activity in human tumor cell cultures. FEBS Lett 176: 479–483, 1984
Münstermann U, Fritz G, Seitz G, Yiping L, Schneider HR, Issinger O-G: Casein kinase CK2 is elevated in solid human tumors and rapidly proliferating non-neoplasic tissue. Eur J Biochem 189: 251–257, 1990
Issinger O-G, Boldyreff B: Abnormal casein kinases. Recent Adv Cell Mol Biol 4: 17–22, 1992
Daya-Makin M, Sanghera JS, Mogentale TL, Lipp M, Parchomchuk J, Hogg JC, Pelech SL: Activation of a tumor-associated protein kinase (p4OTAK) and casein kinase 2 in human squamous cell carcinomas and adenocarcinomas of the lung. Cancer Res 54: 2262–2268, 1994
Faust RA, Gapany M, Tristani P, Davis A, Adams GL, Ahmed K: Elevated protein kinase CK2 activity in chromatin of head and neck tumors: Association with malignant transformation. Cancer Left 101: 31–35, 1996
Brunati AM, Saggioro D, Chieco-Bianchi L, Pinna LA: Altered protein kinase activities of lymphoid cells transformed by Abelson and Moloney leukemia viruses. FEBS Lett 206: 59–63, 1986
Ole-MoiYoi OK, Brown WC, Iams KP, Nayar A, Tsukamoto T, Macklin M: Evidence for the induction of casein kinase II in bovine lymphocytes transformed by the intracellular protozoan parasite Theileria parva. EMBO J 12: 1621–1631, 1993
Seldin DC, Leder P: Casein kinase IIα transgene-induced murine lymphoma: Relation to theilehosis in cattle. Science 267: 894–897, 1995
Roig J, Krehan A, Colomer D, Pyerin W, Itarte E, Plana M: Defined CK2α degradation is a result of specific protease activity. Submitted.
Stuart DI, Jones EY: Cuffing complexity down to size. Nature 386: 437–438, 1997
Krehan A, Lorenz P, Plana-Coll M, Pyerin W: Interaction sites between catalytic and regulatory subunits in human protein kinase CK2 holoenzymes as indicated by chemical crosslinking and immunological investigations. Biochemistry 35: 4966–4975
Kumatori A, Tanaka K, Inamura N, Sone SOT, Matsumoto T, Tachikawa T, Shin S, Ichihara A: Abnormally high expression of proteasomes in human leukemic cells. Proc Natl Acad Sci USA 87: 7071–7075, 1990
Odno E, Tanaka K, Tamura T, Sone S, Ogura T, Ichihara A: ATPdependent reversible association of proteasomes with multiple protein components to form 26 S complexes that degrade ubiquitinated proteins in human HL-60 cells. FEBS Lett 284: 206–210, 1991
Laemmli UK: Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685, 1970
Alba FJ, Bermudez A, Bartolomé S, Daban JR: Detection of five nanograms of protein by two-minute nile red staining of unfixed SDS gels. BioTechniques 21: 615–626, 1996
Tsukahara T, Ishiura S, Sugita H: An ATP-dependent protease and ingensin, the multicatalytic proteinase, in K562 cells. Eur J Biochem 177: 261–266, 1988
Pancetti F, Bosser R, Itarte E, Bachs O: Biochem Biophys Res Commun 218: 35–39, 1996
Antonelli M, Daniotti JL, Rojo D, Allende CC, Allende JE: Cloning, expression and properties of the α′ subunit of casein kinase 2 from zebrafish (Danio rerio). Eur J Biochem 241: 272–279, 1996
Orlowski M: Biochemistry 29: 10289–10297, 1990
Allende JE, Allende CC: Protein kinase CK2: An enzyme with multiple substrates and a puzzling regulation. FASEB J 9: 313–323, 1995
Hériché J-K, Lebrin F, Rabilloud T, Leroy D, Chambaz EM, Goldberg Y: Regulation of protein phosphatase 2A by direct interaction with casein kinase 2α. Science 276: 952–955, 1997
Chen M, Li D, Krebs EG, Cooper JA: The casein kinase 11 beta subunit binds to Mos and inhibits Mos activity. Mol Cell Biol 4: 1904–1912, 1997
Hagemann C, Kalmes A, Wixier V, Wixier L, Schuster T, Rapp UR: The regulatory subunit of protein kinase CK2 is a specific A-Raf activator. FEBS Lett 403: 200–202, 1997
Woods CM, Lazarides E: Degradation of unassembled alpha-and beta-spectrin by distinct intracellular pathways: Regulation of spectrin topogenesis by beta-spectrin degradation. Cell 40: 959–969, 1985
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Roig, J., Krehan, A., Colomer, D. et al. Multiple forms of protein kinase CK2 present in leukemic cells: In vitro study of its origin by proteolysis. Mol Cell Biochem 191, 229–234 (1999). https://doi.org/10.1023/A:1006808816770
Issue Date:
DOI: https://doi.org/10.1023/A:1006808816770