Abstract
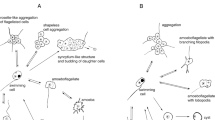
Predation was a powerful selective force promoting increased morphological complexity in a unicellular prey held in constant environmental conditions. The green alga, Chlorella vulgaris, is a well-studied eukaryote, which has retained its normal unicellular form in cultures in our laboratories for thousands of generations. For the experiments reported here, steady-state unicellular C. vulgaris continuous cultures were inoculated with the predator Ochromonas vallescia, a phagotrophic flagellated protist (‘flagellate’). Within less than 100 generations of the prey, a multicellular Chlorella growth form became dominant in the culture (subsequently repeated in other cultures). The prey Chlorella first formed globose clusters of tens to hundreds of cells. After about 10–20 generations in the presence of the phagotroph, eight-celled colonies predominated. These colonies retained the eight-celled form indefinitely in continuous culture and when plated onto agar. These self-replicating, stable colonies were virtually immune to predation by the flagellate, but small enough that each Chlorella cell was exposed directly to the nutrient medium.
Similar content being viewed by others
References
Abrams, P.A. (1986) Adaptive responses of predators to prey and prey to predators: The failure of the armsrace analogy. Evolution 40, 1229–1247.
Bakker, R.T. (1983) The deer flees, the wolf pursues: Incongruencies in predator-prey evolution. In Coevolution (D.J. Futuyma and M. Slatkin, eds), pp. 350-382. Sinauer Associates, Sunderland, MA.
Bennett, W.N. and Boraas, M.E. (1989) A demographic profile of the fastest growing metazoan: A strain of Brachionus calyciflorus Pallas (Rotifera). Oikos 55, 365–369.
Bennett, W.N., Boraas, M.E. and Seale, D.B. (1993) Turbidostat culture of Brachionus calyciflorus: An experimental system to assess biological limits on population growth. In Plankton Regulation Dynamics(N. Walz, ed.), pp. 77–86. Springer-Verlag, Berlin.
Bonner, J.T. (1988) The Evolution of Complexity by Means of Natural Selection. Princeton University Press, Princeton, NJ.
Boraas, M.E. (1979) Dynamics of nitrate, algae, and rotifers in continuous culture: Experiments and model simulations. PhD thesis, The Pennsylvania State University, State College, PA.
Boraas, M.E. (1980) A chemostat system for the study of rotifer-algal-nitrate interactions. In Evolution and Ecology of Zooplankton Communities (W.C. Kerfoot, ed.), pp. 173–182. University Press of New England, Hanover, NH.
Boraas, M.E. (1983) Population dynamics of food-limited rotifers in two-stage chemostat culture. Limnol. Oceanogr. 28, 546–563
Boraas, M.E., Estep, K.W., Johnson, P.W. and Sieburth, J.McN. (1988) Phagotrophic phototrophs: The ecological significance of mixotrophy. J. Protozool. 35, 249–252.
Boraas, M.E., Seale, D.B. and Holen, D. (1992) Predatory behavior of Ochromonas analyzed with video microscopy. Arch. Hydrobiol. 123, 459–468.
Brodie, E.D., III and Brodie, E.D., Jr (1990) Tetrodotoxin resistance in garter snakes: An evolutionary response of predators to dangerous prey. Evolution 44, 651–659.
Brodie, E.D., III and Brodie, E.D., Jr (1991) Evolutionary response of predators to dangerous prey: Reduction of toxicity of newts and resistance of garter snakes in island populations. Evolution 45, 221–224.
Chao, L., Levin, B.R. and Stewart, F.M. (1977) A complex community in a simple habitat: An experimental study with bacteria and phage. Ecology 58, 369–378.
Dawkins, R. and Krebs, J.R. (1979) Arms races between and within species. Proc. R. Soc. Lond. B 205, 489–511.
Dodson, S.I. (1989) The ecological role of chemical stimuli for the zooplankton: Predator-induced morphology in Daphnia. Oecologia 78, 361–367.
Fott, B. and Novakova, M. (1969) A monograph of the genus Chlorella, the freshwater species. In Studies in Phycology (B. Fott, ed.), pp. 10–74. E. Schweizerbart’sche Verlagsbuchhandlung, Stuttgart.
Gillott, M., Holen, D., Ekman, J., Harry, M. and Boraas, M. (1993) Predation-induced E. coli filaments: Are they multicellular? In Proceedings of the 51st Annual Meeting of the Microscopy Society of America (G. Baily and C. Reider, eds), p. 420. San Francisco Press, San Francisco, CA.
Gould, S.J. (1975) A direct assault upon the citadel itself. Paleobiology 1, 125–129.
Gray, M.W. and Doolittle, W.F. (1982) Has the endosymbiotic hypothesis been proven? Microbiol. Rev. 46, 1–42.
Hanson, E.D. (1977) The Origin and Early Evolution of Animals. Wesleyan University Press, Middletown, CT.
Hessen, D.O. and Van Donk, E. (1993) Morphological changes in Scenedesmus induced by substances released from Daphnia. Arch. Hydrobiol. 127, 129–140.
Holen, D.A. and Boraas, M.E. (1991) The feeding behavior of Spumella sp. as a function of particle size: Implications for bacterial size in pelagic systems. Hydrobiologia 220, 73–88.
Holen, D.A. and Boraas, M.E. (1995) Mixotrophy in chrysophytes. In Chrysophyte Algae: Ecology, Phylogeny and Development (C. Sandgren, J.P. Smol and J. Kristiansen, eds), pp. 119–140. Cambridge University Press, Cambridge.
Hori, M. (1993) Frequency dependent natural selection in the handedness of scale-eating cichlid fish. Science 260, 216–219.
Jerison, H.J. (1970) Brain evolution: New light on old principles. Science 170, 1224–1225.
Jerison, H.J. (1973) Evolution of the Brain and Intelligence. Academic Press, New York.
Margulis, L. (1981) Symbiosis in Cell Evolution. W.H. Freeman, San Francisco, CA.
Paine, R.T. (1966) Food web complexity and species diversity. Am. Nat. 100, 65–75.
Radinsky, L. (1978) Evolution of brain size in carnivores and ungulates. Am. Nat. 112, 815–831.
Schaffer, W.N. and Rosenzweig, M.L. (1978) Homage to the Red Queen. I. Coevolution of predators and their victims. Theor. Pop. Biol. 14, 135–157.
Seale, D.B. (1980) Influence of amphibian larvae on primary production, nutrient flux, and competition in a pond ecosystem. Ecology 61, 1531–1550.
Seale, D.B. (1982) Obligate and facultative suspension feeding in anuran larvae: Feeding regulation in Xenopusand Rana . Biol. Bull. 162, 214–231.
Seale, D.B., Boraas, M.E., Holen, D.A. and Nealson, K.H. (1990) Use of bioluminescent bacteria, Xenorhabdus luminescens, to measure predation by freshwater microflagellates. FEMS Microb. Ecol. 73, 31–40.
Shikano, S., Luckinbill, L.S. and Kurihara, Y. (1990) Changes of traits in a bacterial population associated with protozoal predation. Microb. Ecol. 20, 75–84.
Stanley, S.M. (1973) An ecological theory for the sudden origin of multicellular life in the Late Precambrian. PNAS 70, 1486–1489.
Stephens, D.W. and Krebs, D.W. (1986) Foraging Theory. Princeton University Press, Princeton, NJ.
Thompson, J.N. (1982) Interaction and Coevolution. John Wiley, New York.
Tilman, D. (1982) Resource Competition and Community Structure. Princeton University Press, Princeton, NJ.
Townsend, C.R. and Calow, P. (1981) Physiological Ecology: An Evolutionary Approach to Resource Use. Sinauer Associates, Sunderland, MA.
Van Valen, L. (1973) A new evolutionary law. Evol. Theory 1, 1–30.
Vermeij, G.J. (1987) Evolution and Escalation: An Ecological History of Life. Princeton University Press, Princeton, NJ.
Weis, A.E., McCrea, K.D. and Abrahamson, W.G. (1989) Can there be an escalation arms race without coevolution? Implications from a host-parasitoid simulation. Evol. Ecol. 3, 361–370.
Williams, F.M. (1971) Dynamics of microbial populations. In Systems Analysis and Simulation in Ecology(B.C. Patten, ed.), pp. 197–267. Academic Press, New York.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Boraas, M.E., Seale, D.B. & Boxhorn, J.E. Phagotrophy by a flagellate selects for colonial prey: A possible origin of multicellularity. Evolutionary Ecology 12, 153–164 (1998). https://doi.org/10.1023/A:1006527528063
Issue Date:
DOI: https://doi.org/10.1023/A:1006527528063