Abstract
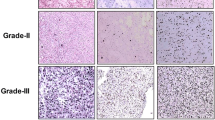
Cumulative inactivation of tumor suppressor genes and/or amplification of oncogenes lead to progressively more malignant astrocytic tumors. We have analyzed the significance of tumor suppressor genes p53, p21, p16 and retinoblastoma protein (pRb) and proliferative activity for survival in 77 high grade astrocytic tumors.
After operation, the patients – 25 anaplastic astrocytomas (AA) and 52 glioblastomas (GBs) – were treated with similar radiotherapy. The expression of the suppressor genes and the proliferative activity were analyzed immunohistochemically.
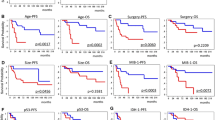
p53 immunopositivity was found in 44% of AAs and 46% of GBs. Tumors with aberrant p53 expression had lower proliferation indices than p53 immunonegative tumors. Neither p53 expression nor p21 immunonegativity (52% of AAs and 48% of GBs) correlated with survival. p16 immunostaining was negative in 16% of AAs and in 44% of GBs, and it correlated inversely with survival in both uni- and multivariate analyses. pRb immunostaining was negative only in 8% of both AAs and GBs and the absence of p16 and pRb were mutually exclusive.
Ki-67 labelling index (LI) was significantly higher in GBs (26.8%) than in AAs (20.3%), and in multivariate analysis it was an independent prognostic factor for survival. In 48% of AAs Ki-67 LI exceeded 20% and this subset of AAs had similar prognosis as GB.
In high grade astrocytic tumors p16 immunonegativity was an independent indicator of poor prognosis in addition to the previously established patient's age, histopathology and Ki-67 LI. Furthermore, there was a subset of AAs with a high proliferation rate (>20%) in which the histopathological hallmarks of GB were lacking, but which had similarly dismal prognosis as GB.
Similar content being viewed by others
References
Salminen E, Nuutinen J, Huhtala S: Multivariate analysis of prognostic factors in 106 patients with malignant glioma. Eur J Cancer 32: 1918-1923, 1996
Bleehen N, Stenning S: A medical research council trial of two radiotherapy doses in the treatment of grades 3 and 4 astrocytoma. The Medical Research Council Brain Tumour Working Party. Br J Cancer 64: 769-774, 1991
Kleihues P, Burger PC, Scheithauer BW: Histological typing of tumors of the central nervous system. In: World Health Organization International Histological Classification of Tumors. Springer Verlag, Berlin, Heidelberg, 1993
James C, Olson J: Molecular genetics and molecular biology advances in brain tumors. Curr Opinion Onc 8: 188-195, 1996
Sallinen P, Haapasalo H, Visakorpi T, Helen PT, Rantala IS, Isola JJ, Helin HJ: Prognostication of astrocytoma patient survival by Ki-67 (MIB-1), PCNA, and S-phase fraction using archival paraffin-embedded samples. J Pathol 174: 275-282, 1994
Gerdes J, Li L, Schlueter C, Duchrow MC, Wohlenberg C, Gerlach C: Immunobiochemical and molecular biologic characterization of the cell proliferation associated nuclear antigen that is defined by monoclonal antibody Ki-67. AmJ Pathol 138: 867-873, 1991
Cattoretti G, Becker MHG, Key G, Duchrow M, Schluter C, Galle J, Gerdes J: Monoclonal antibodies against recombinant parts of the Ki-67 antigen (MIB-1 and MIB-3) detect proliferating cells in microwave-processed formalin-fixed paraffin-embedded sections. J Pathol 168: 357-363, 1992
Burger PC, Shibata T, Kleihues P: The use of monoclonal antibody Ki-67 in the identification of proliferating cells. Surg Pathol 10: 611-617, 1986
Geradts J, Kratzke R, Niehans G, Wohlenberg C, Gerlach C: Immunohistochemical detection of the cyclin-dependent kinase inhibitor 2/multiple tumor suppressor gene 1 (CDKN2/MTS1) product p16INK 4A in archival human solid tumors, correlation with retinoblastoma protein expression. Cancer Res 55: 6006-6011, 1995
Louis D: A molecular genetic model of astrocytoma histopathology. Brain Pathol 7: 755-764, 1997
Jen J, Harper J, Bigner S, Bigner D, Papadopoulos N, Markowitz S, Willson J, Kintzer K, Vogelstein B: Deletion of p16 and p15 genes in brain tumors. Cancer Res 54: 6353-6358, 1994
He J, Olson J, James C: Lack of p16INK4 or retinoblastoma protein (pRb), or amplification-associated overexpression of cdk4 is observed in distinct subsets of malignant glial tumors and cell lines. Cancer Res 55: 4833-4836, 1995
Nishikawa R, Furnari F, Lin H, Arap W, Berger M, Cavenee W, Huang H-J: Loss of p16INK4 expression is frequent in high grade gliomas. Cancer Res 55: 1941-1945, 1995
Tsuzuki T, Tsunoda S, Sakaki T, Konishi N, Hiasa Y, Nakamura M: Alterations of retinoblastoma, p53, p16(CDKN2), and p15 genes in human astrocytomas. Cancer 78: 287-293, 1996
Perry A, Nobori T, Ru N, Anderl K, Borell T, Mohapatra G, Feuerstein B, Jenkins R, Carson D: Detection of p16 gene deletions in gliomas, a comparision of fluorescence in situ hybridization (FISH) versus quantitative PCR. J Neuropathol Exp Neurol 56: 999-1008, 1997
Louis D: The p53 gene and protein in human brain tumors. J Neuropathol Exp Neurol 53: 11-21, 1994
Ellison DW, Steart PV, Bateman AC, Pickering RM, Palmer JD, Weller RO: Prognostic indicators in a range of astrocytic tumors: an immunohistochemical study with Ki-67 and p53 antibodies. J Neurol Neurosurg Psychiatry 59: 413-419, 1995
Zuber P, Hamou MF, de Tribolet N: Identification of proliferating cells in human gliomas using the monoclonal antibody Ki-67. Neurosurgery 22: 364-368, 1988
Haapasalo H, Isola J, Sallinen P, Kalimo H, Helin H, Rantala I: Aberrant p53 expression in astrocytic neoplasms of the brain, association with proliferation. Am J Pathol 142: 1347-1351, 1993
Wakimoto H, Aoyagi M, Nakayama T, Nagashima G, Yamamoto S, Tamaki M, Hirakawa K: Prognostic significance of Ki-67 labelling indices obtained using MIB-1 monoclonal antibody in patients with supratentorial astrocytomas. Cancer 77: 373-380, 1996
Hsu D, Louis D, Efird J, Hedley-Whyte E: Use of MIB-1 (Ki-67) immunoreactivity in differentiating grade II and grade III gliomas. J Neuropathol Exp Neurol 56: 857-865, 1997
Bögler O, Huang HJ, Kleihues P, Cavenee WK: The p53 gene and its role in human brain tumors. Glia 15: 308-327, 1995
von Deimling A, von Ammon K, Schoenfend D, Wiestler OD, Seizinger BR, Louis DN: Subsets of glioblastoma multiforme defined by molecular genetic analysis. Brain Pathol 3: 19-26, 1993
Lang F, Miller D, Koslow M, Newcomb E: Pathways leading to glioblastoma multiforme, a molecular analysis of genetic alterations in 65 astrocytic tumors. J Neurosurg 81: 427-436, 1994
Reifenberger J, Ring G, Gies U, Cobbers J, Obertrass J, An H, Niederacher D, Wechsler W, Reifenberger G: Analysis of p53 mutation and epidermal growth factor receptor amplification in recurrent gliomas with malignant progression. J Neuropathol Exp Neurol 55: 822-831, 1996
Kleihues P, Ohgaki H: Genetics of glioma progression and the definition of primary and secondary glioblastoma. Brain Pathol 7: 1131-1136, 1997
Watanabe K, Tachibana O, Sato K, Yonekawa Y, Kleihues P, Ohgaki H: Overexpression of the EGF receptor and p53 mutations are mutually excusive in the evolution of primary and secondary glioblastomas. Brain Pathol 6: 217-224, 1996
Louis D, von Deimling A, Chung R, Rubio M, Whaley J, Eibl R, Ohgaki H, Wiestler O, Thor A, Seizinger BR: Comparative study of p53 gene and protein alterations in human astrocytic tumors. J Neuropathol Exp Neurol 52: 31-38, 1993
Newcomb EW, Madonia WJ, Pisharody S, Lang FF, Koslow M, Miller DC: A correlative study of p53 protein alteration and p53 gene mutation in glioblastoma multiforme. Brain Pathol 3: 229-235, 1993
Moulton T, Samara G, Chung W, Yuan L, Desai R, Sisti M, Bruce J, Tycko B: MTS1/p16/CDKN2 lesions in primary glioblastoma multiforme. Am J Pathol 146: 613-619, 1995
Ueki K, Ono Y, Henson J, Efird J, von Deimling A, Louis D: CDKN2/p16 or RB alterations occur in the majority of glioblastomas and are inversely correlated. Cancer Res 56: 150-153, 1996
Piva R, Cavalla P, Bortolotto S, Grosso R, Richiardi P, Dutto A, Schiffer D: CDKN2/p16 inactivation and p16 immunohistochemistry in astrocytic gliomas. Int J Oncol 12: 55-58, 1998
Nakamura M, Konishi M, Hiasa Y, Tsunoda S, Fukushima Y, Tsuzuki T, Takemura K, Aoki H, Kobitsu K, Sakaki T: Immunohistochemical detection of CDKN2, retinoblastoma and p53 gene products in primary astrocytic tumors. Int J Oncol 8: 889-893, 1996
Rao LS, Miller DC, Newcomb EW: Correlative immunohistochemistry and molecular genetic study of the inactivation of the p16INK4A genes in astrocytomas. Diagn Mol Pathol 6: 115-122, 1997
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kirla, R., Salminen, E., Huhtala, S. et al. Prognostic Value of the Expression of Tumor Suppressor Genes p53, p21, p16 and pRb, and Ki-67 Labelling in High Grade Astrocytomas Treated with Radiotherapy. J Neurooncol 46, 71–80 (2000). https://doi.org/10.1023/A:1006473320474
Issue Date:
DOI: https://doi.org/10.1023/A:1006473320474