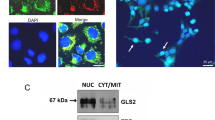
Abstract
In transformed human glial cells, abnormalities of the p53 gene and altered expression of glial-specific properties (GSPs) have been observed. We therefore investigated whether (i) expression of the altered p53 protein is involved in the reduced expression of GSPs; and (ii) expression of the wild-type p53 (wt-p53) gene leads to induction of GSPs. We first determined that the p53 gene is mutated in human glioblastoma U-373MG cells. In these cells, and in human T-98G glioblastoma cells reported to possess a mutated p53 (m-p53) gene, nuclear m-p53 expression was intense while GSP expression was low in the same cell as revealed by double labelling immunocytochemistry. Conversely, glial fibrillary acidic protein (GFAP) and glutamate synthase (GS) were expressed in cells devoid of nuclear m-p53 immnunoreactivity. Therefore, a mutually exclusive relationship exists between the cytoplasmic GSPs and nuclear m-p53. Upon treatment with retinoic acid (RA) and dibutyryl cyclic AMP (dbcAMP), overall GSP staining were increased concomitant with suppression of nuclear m-p53. Their mutually exclusive expression pattern was maintained suggesting a functional relationship. This is supported by the observation of a similar mutually exclusive expression pattern for p53 and GSPs in pathologic specimens of human glioblastoma tissues.
We then explored the role of the wt-p53 gene in the induction of GSPs using a wt-p53 tetracycline-regulated conditional expression system in human LN-Z308 glioblastoma cells. These cells normally express no p53 and no appreciable levels of GS or GFAP. Induced expression of wt-p53 lead to induction of GSP. These observations are consistent with the hypotheses that (i) nuclear m-p53 expression and cytoplasmic expression of GFAP and GS are inversely correlated, and (ii) expression of the wt-p53 gene leads to the expression of GSPs.
Similar content being viewed by others
References
Bignami A, Dahl D: Differentiation of astrocytes in the cerebellar cortex and the pyramidal tracts of the newborn rat. An immunofluorescence study with antibodies to a protein specific to astrocytes. Brain Res 49: 393-402, 1973
Haynes LW, Weller RO: Induction of some features of glial differentiation in primary culture of human gliomas by treatment with dibutyryl cyclicAMP. Br J Exp Path 59: 259-276, 1978
Liwnicz BH, Archer G, Soukup SW, Liwnicz RG: Continuous human glioma-derived cell lines UC-11MG and UC-302MG. J Neuro-Oncology 3: 373-385, 1986
Ponten J, Westermark B: Properties of human malignant glioma cells in vitro. Med Biol 56: 184-193, 1978
Raible, DW, McMorris FA: Cyclic AMP regulates the rate of differentiation of oligodendrocytes without changing the lineage commitment of their progenitors. Dev Biol 133: 437-446, 1989
Raible DW, McMorris FA: Induction of oligodendrocyte differentiation by activators of adenylate cyclase. J Neurosci Res 27: 43-46, 1990
Arcuri C, Bocchini V, Guerrieri P et al.: PKA and PKC activation induces opposite glial fibrillary acidic protein (GFAP) expression and morphology changes in a glioblastoma multiform cell line of clonal origin. J Neurosci Res 40: 622-631, 1995
Kajiwara K, Orita T, Nishizake T et al.: Glial fibrillary acidic protein (GFAP) expression and nucleolar organizer regions (NORs) in human gliomas. Brain Res 572: 314-318, 1992
Bischoff JR, Friedman PN, Marshak DR, Prives C, Beach D: Human p53 is phosphorylated by p60-cdc2 and cyclin B-cdc2. Proc Natl Acad Sci 87: 4766-4770, 1990
Chen PL, Scully P, Shew JY, Wang JYJ, and Lee WH: Phosphorylation of the retinoblastoma gene product is modulated during the cell cycle and cellular differentiation. Cell 58: 1193-1198, 1989
Ludlow JW, Shon J, Pipas JM, Livingston DM, DeCaprio JA: The retinoblastoma susceptibility gene product undergoes cell cycle-dependent phosphorylation and binding to and release from SV40 large T. Cell 60: 387-396, 1990
Marshall CJ: Oncogenes and growth control 1987. Cell 49: 723-725, 1987
Mercer WE, Shields MT, Aurin M, Sauve GJ, Appella E, Romano JW and Ullrich SJ: Negative growth regulation in a glioblastoma tumor cell line that conditionally expresses human wild-type p53. Proc Natl Acad Sci 87: 6166-6170, 1990
Raycroft L, Wu H, Lozano G: Transcriptional activation by wild-type but not transforming mutants of the p53 antioncogene. Science 249: 1049-1051, 1990
U HS, Kelley PY, Hatton JD, Shew JY: Proto-oncogene abnormalities and their relationship to tumorigenicity in some human glioblastomas. J Neurosurg 71: 83-90, 1989
Yahanda AM, Bruner JM, Donehower LA, Morrison RS: Astrocytes derived from p53-deficient mice provide a multistep in vitro model for development of malignant gliomas. Mol Cell Biol 15: 4249-4259, 1995
Baker SJ, Fearson ER, Nigro JM, Hamilton SR, Preisinger AC, Jessup JM, van Tuinen P, Ledbetter DH, Barker DF, Nakamura Y et al.: Chromosome 17 deletions and p53 gene mutations on colorectal carcinomas. Science 244: 217-221, 1989
Malkin D, Li FP, Strong LC, Fraumeni Jr JF, Nelson CE, Kim DH, Kassel J, Gryka M, Bischoff FZ, Tainsky MA, Friend SH: Germ line p53 mutations in a familial syndrome of breast cancer, sarcomas, and other neoplasms. Science 250: 1233-1238, 1990
Nigro JM, Baker SJ, Preisinger AC, Jessup JM, Hostetter R, Cleary K, Bigner SH, Davidson N, Baylin S, Devilee P, Glover T, Collins FS, Weston A, Modali R, Harris CC, Vogelstein B: Mutations in the p53 gene occur in diverse human tumour types. Nature 342: 705-708, 1989
Eliyahu D, Raz A, Gruss P, Givol D, Oren M: Participation of p53 cellular tumour antigen in transformation of normal embryonic cells. Nature 312: 646-649, 1984
Halvey O, Michalovitz D, Oren M: Different tumor-derived p53 mutants exhibit distinct biological activities. Science 250: 113-116, 1990
Hupp TR, Meek DW, Midgley CA, Lane DP: Regulation of the specific DNA binding function of p53. Cell 71: 875-886, 1992
Parada LF, Land H, Weinberg RA, Wolf D, Rotter V: Cooperation between gene encoding p53 tumour antigen and ras in cellular transformation. Nature 312: 649-651, 1984
Reich NC, Oren M, Levine AJ: Two distinct mechanisms regulate the levels of a cellular tumor antigen, p53. Mol Cell Biol 3: 2143-2150, 1983
Reihsaus E, Kohler M, Kraiss S, Oren M, Montenarh M: Regulation of the level of the oncoprotein p53 in nontransformed and transformed cells. Oncogene 5: 137-145, 1990
Rogel A, Popliker M, Webb CG, Oren M: p53 cellular tumor antigen: analysis of mRNA levels in normal adult tissues, embryos, and tumors. Mol Cell Biol 5: 2851-2855, 1985
Shaulsky G, Goldfinger N, Ben-Ze'er A, Rotter V: Nuclear accumulation of p53 protein is mediated by several nuclear localization signals and plays a role in tumorigenesis. Mol Cell Biol 10: 6565-6577, 1990
Chen PL, Chen Y, Bookstein R, Lee WH: Genetic mechanisms of tumor suppression by the human p53 gene. Science 250: 1576-1580, 1990
Sidell N, Koeffler HP: Modulation of Mr 53,000 protein with induction of differentiation of human neuroblastoma cells. Proc Natl Acad Sci 48: 2226-2230, 1988
Steinman RA, Hoffman B, Iro A, Guillouf C, Lieberman DA, El-Houseini M: Induction of p21 (WAR-1/CIP1) during differentiation. Oncogene 9: 3389-3396, 1994
Nadal A, Jares P, Cazorla M, Fernandez PL, Sanjuan X, Hernandez L, Pinyol M, Aldea M, Mallofre C, Muntane J, Traserra J, Campo E, Cardesa A: p21WAP1/Cip1 expression is associated with cell differentiation but not with p53 mutations in squamous cell carcinomas of the larynx. J Path 183: 156-163, 1997
Kokunai T, Izawa I, Tamaki N: Overexpression of p21WAP1/Cip1 induces cell differentiation and growth inhibition in a human glioma cell line. Int J Cancer 75: 643-648, 1998
Khochbin S, Principaud E, Chabanas A, and Lawrence J-J: Early events in murine erythroleukemia cells induced to differentiate. J Mol Biol 200: 55-64, 1988
Khochbin S, Lawrence J-J: An antisense RNA involved in p53 mRNA maturation in murine erythroleukemia cells induced to differentiate. EMBO J 8: 4107-4114, 1989
Klinken SP, Holmes KL, Morse III HC, Thorgeirsson SS: Transcriptional and post-transcriptional regulation of c-myc, c-myb, and p53 during proliferation and differentiation of murine erythroleukemia cells treated with DFMO and DMSO. Exp Cell Res 178: 185-198, 1988
Richon VM, Ramsay RG, Rifkind RA, Marks PA: Modulation of the c-myb, c-myc and p53 mRNA and protein levels during induced murine erythroleukemia cell differentiation. Oncogene 4: 165-173, 1989
Milner J, Medcalf EA, Cook AC: Tumor suppressor p53: analysis of wild-type and mutant p53 complexes. Mol Cell Biol 11: 12-19, 1991
Van Meir EG, Polverini PJ, Chazin VR, Huang HJ Su, de Tribolet N, Cavenee WK: Relaese of an inhibitor of angiogenesis upon induction of wild type p53 expression in glioblastoma cells. Nature Gen 8: 171-176, 1994
Tabuchi I, Fukuyama K, Mineta T: Cytogenetic diagnosis of brain tumors. Brain Nerv 44: 871-879, 1992
Hollstein M, Sidransky D, Vogelstein B, Harris CC: p53 mutations in human cancers. Science 253: 49-53, 1991
Lamb P, Crawford L: Characterization of the human p53 gene. Mol Cell Biol 6: 1379-1385, 1985
Sanger F, Nicklen F, Coulsen AR: DNA sequencing with chain terminating inhibitors. Proc Natl Acad Sci 74: 5463-5467, 1977
U HS, Espiritu OD, Kelley PY, Klauber MR, Hatton JD: The role of the epidermal growth factor receptor (EGF-R) in human gliomas: II. The control of cell growth. J Neurosurg 82: 841-846, 1995
U HS, Espiritu OD, Kelley PY, Klauber MR, Hatton JD: The role of the epidermal growth factor receptor (EGF-R) in human gliomas: II. The control of glial process extension and the expression of glial fibrillary acidic protein (GFAP). J Neurosurg 82: 847-857, 1995
Norenberg MD, Neary JT, Norenberg LB et al.: Ammonia induced decrease in glial fibrillary acidic protein in cultured astrocytes. J Neuropath Exp Neurol 49: 399-405, 1990
Muir D, Varon S, Manthorpe M: An enzyme-linked immunosorbent assay for bromodeoxyuridine incorporation using fixed microcultures. Analytical Biochem 185: 377-382, 1990
Ullrich SJ, Mercer WE, Appella E: Human wild-type p53 adopts a unique conformational and phosphorylation state in vivo during growth arrest of glioblastoma cells. Oncogene 7: 1635-1643, 1992
Gu Y, Turck CW, Morgan DO: Inhibition of CDK2 activity in vivo by an associated 20K regulatory subunit. Nature 366: 707-710, 1993
Martinez J, Georgoff I, Martinez J, Levine AJ: Cellular localization and cell cycle regulation by a temperature-sensitive p53 protein. Genes Develop 5: 151-159, 1991
Gannon JV, Lane DP: Protein synthesis required to anchor a mutant p53 protein which is temperature-sensitive for nuclear transport. Nature 349: 802-806, 1991
Ginsberg D, Michael-Michalovitz D, Ginsberg D, Oren M: Induction of growth arrest by a temperature-sensitive p53 mutant is correlated with increased nuclear localization and decrease stability of the protein. Mol Cell Biol 11: 582-585, 1991
Shaulsky G, Goldfinger N, Peled A, Rotter V: Involvement of wild-type p53 protein in the cell cycle requires nuclear localization. Cell Growth Develop 2: 661-667, 1991
Shaulsky G, Goldfinger N, Rotter V: Alterations in tumor development in vivo mediated by expression of wild type or mutant p53 proteins. Cancer Res 51: 5232-5237, 1991
Fields S, Jang SK: Presence of a potent transcription activating sequence in the p53 protein. Science 249: 1046-1048, 1990
El-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, Lin D, Mercer WE, Kinzler KW, Vogelstein B: WAF1, a potential mediator of p53 tumor suppression. Cell 75: 817-825, 1993
Harper JW, Adami GR, Wei N, Keyomarsi K, Elledge SJ: The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell 75: 805-816, 1993
Xiong Y, Hannon GJ, Zhang H, Casso D, Kobayashi R, Beach D: p21 is a universal inhibitor of cyclin kinases. Nature 366: 701-704, 1993
Deng C, Zhang P, Harper JW, Elledge SJ, Leder P: Mice lacking p21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell 82: 675-684, 1995
Weinberg RA: The retinoblastoma protein and cell cycle control. Cell 81: 323-330, 1995
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sang U, H., Banaie, A., Rigby, L. et al. Alteration in p53 Modulates Glial Proteins in Human Glial Tumour Cells. J Neurooncol 48, 191–206 (2000). https://doi.org/10.1023/A:1006453316656
Issue Date:
DOI: https://doi.org/10.1023/A:1006453316656