Abstract
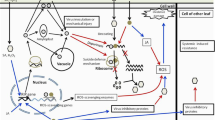
Pokeweed antiviral protein (PAP), a ribosome-inactivating protein isolated from Phytolacca americana, is characterized by its ability to depurinate the sarcin/ricin (S/R) loop of the large rRNA of prokaryotic and eukaryotic ribosomes. In this study, we present evidence that PAP is associated with ribosomes and depurinates tobacco ribosomes in vivo by removing more than one adenine and a guanine. A mutant of pokeweed antiviral protein, PAPn, which has a single amino acid substitution (G75D), did not bind ribosomes efficiently, indicating that Gly-75 in the N-terminal domain is critical for the binding of PAP to ribosomes. PAPn did not depurinate ribosomes and was non-toxic when expressed in transgenic tobacco plants. Unlike wild-type PAP and a C-terminal deletion mutant, transgenic plants expressing PAPn did not have elevated levels of acidic pathogenesis-related (PR) proteins. PAPn, like other forms of PAP, did not trigger production of salicylic acid (SA) in transgenic plants. Expression of the basic PR proteins, the wound-inducible protein kinase and protease inhibitor II, was induced in PAPn-expressing transgenic plants and these plants were resistant to viral and fungal infection. These results demonstrate that PAPn activates a particular SA-independent, stress-associated signal transduction pathway and confers pathogen resistance in the absence of ribosome binding, rRNA depurination and acidic PR protein production.
Similar content being viewed by others
References
Aron, G. and Irvin, J.D. 1980. Inhibition of herpes simplex virus multiplication by the pokeweed antiviral protein. Antimicrob. Ag. Chemother. 17: 1032–1033.
Balandin, T., van der Does, C., Albert, J.-M.B., Bol, J.F. and Linthorst, H.J.M. 1995. Structure and induction pattern of a novel proteinase inhibitor class II gene of tobacco. Plant Mol. Biol. 27: 1197–1204.
Barbieri, I., Aron, G.M., Irvin, J.D. and Stirpe, F. 1982. Purification and partial characterization of another form of the antiviral protein from the seeds of Phytolacca americana L. (pokeweed). Biochem. J. 203: 55–59.
Bonness, M.B., Ready, M.P., Irvin, J.D. and Mabry, T.J. 1994. Pokeweed antiviral protein inactivates pokeweed ribosomes: implications for the antiviral mechanism. Plant J. 5: 173–183.
Chen, Z.C., White, R.F., Antoniw, J.F. and Lin, Q. 1991. Effect of pokeweed antiviral protein (PAP) on the infection of viruses. Plant Path. 40: 612–620.
Dangl, J.L., Dietrich, R.A. and Richberg, M.H. 1996. Death don't have no mercy: cell death programs in plant-microbe interactions. Plant Cell 8: 1793–1807.
Endo, Y. and Tsurugi, K. 1988. The site of action of six different ribosome-inactivating proteins from plants on eukaryotic ribosomes: the RNA N-glycosidase activity of the proteins. Biochem. Biophys. Res. Commun. 150: 1032–1036.
Green, T.R. and Ryan, C.A. 1972. Wound-induced proteinase inhibitors in plant leaves: a possible defense mechanism against insects. Science 175: 776–777.
Hartley, M.R., Legname, G., Osborn, R., Chen, Z. and Lord, M.J. 1991. Single-chain ribosome-inactivating proteins from plants depurinate Escherichia coli 23S ribosomal RNA. FEBS Lett. 290: 65–1280.
Hemenway, C., Fang, R.-X., Kaniewski, W.K., Chua N.-H. and Tumer, N.E. 1988. Analysis of the mechanism of protection in transgenic plants expressing the potato virus X coat protein or its antisense RNA. EMBO J. 7: 1273–1280.
Hemenway, C., Weiss, J., O'Connell, K. and Tumer, N.E. 1990. Characterization of infectious transcripts of potato virus X cDNA clone. Virology 175: 365–371.
Hudak, K.A., Dinman, J.D. and Tumer, N.E. 1999. Pokeweed antiviral protein accesses ribosomes by binding to L3. J. Biol. Chem. 274: 3859–3864.
Hudak, K.A., Wang, P. and Tumer, N.E. 2000. A novel mechanism for inhibition of translation by pokeweed antiviral protein: depurination of the capped RNA template. RNA 6: 1–12.
Hur, Y., Hwang, D.-J., Zoubenko, O., Coetzer, C., Uckun, F. and Tumer, N.E. 1995. Isolation and characterization of pokeweed antiviral protein mutations in Saccharomyces cerevisiae: identification of residues important for toxicity. Proc. Natl. Acad. Sci. USA 90: 8448–8452.
Irvin, J.D. 1995. Antiviral proteins from Phytolacca. In: Chessin, M., DeBorde, D. and Zipf, A. (Eds.) Antiviral Proteins in Higher Plants, CRC Press, Boca Raton, FL, pp. 65–94.
Irvin, J.D. and Uckun, F.M. 1992. Pokeweed antiviral protein: ribosome inactivation and therapeutic applications. Pharmaceut. Ther. 55: 279–302.
Lodge, J.K., Kaniewski, W.K. and Tumer, N.E. 1993. Broadspectrum virus resistance in transgenic plants expressing pokeweed antiviral protein. Proc. Natl. Acad. Sci. USA 90: 7089–7093.
Malamy, J., Carr, J.P., Klessig, D.F. and Raskin, I. 1990. Salicylic acid: a likely endogenous signal in the resistance response of tobacco to viral infection. Science 250: 1002–1004.
Mittler, R. and Lam, E. 1996. Sacrifice in the face of foes: pathogeninduced programmed cell death in plants. Trends Microbiol. 4: 10–15.
Montanaro, L., Sperti, S., Mattioli, A., Testoni, G. and Stirpe, F. 1975. Inhibition by ricin of protein synthesis in vitro. Biochem. J. 146: 127–131.
Osborn, R.W. and Hartley, M.R. 1990. Dual effects of the ricin A-chain on protein synthesis in C rabbit reticulocyte lysate. Inhibition of initiation and translocation. Eur. J. Biochem. 193: 401–417.
Pieterse, C.M.J., van Weels, S.C.M., Hoffland, E., van Pelt, J.A. and van Loon, L.C. 1996. Systemic resistance in Arabidopsis induced by biocontrol bacteria is independent of salicylic acid accumulation and pathogenesis-related gene expression. Plant Cell 8: 1225–1237.
Pieterse, C.M.J., van Wees, S.C.M., van Pelt, J.A., Knoester, M., Laan, R., Gerrits, H., Weisbeek, P.J. and van Loon, L.C. 1998. A novel signaling pathway controlling induced systemic resistance in Arabidopsis. Plant Cell 10: 1571–1580.
Pieterse, C.M.J. and van Loon, L.C. 1999. Salicylic acidindependent plant defence pathways. Trends Plant Sci. 4: 52–58.
Press, C.M., Wilson, M., Tuzun, S. and Kloepper, J.W. 1997. Salicylic acid produced by Serratia marcescens 90-166 is not the primary determinant of induced systemic resistance in cucumber or tobacco. Mol. Plant-Microbe Interact. 10: 761–768.
Ryals, J.A., Neuenschwander, U.H., Wilits, M.G., Molina, A., Steiner, H.-Y. and Hunt, M. 1996. Systemic acquired resistance. Plant Cell 8: 1809–1819.
Schweizer, P., Buchala, A., Dudler, R. and Metraux, J.-P. 1998. Induced systemic resistance in wounded rice plants. Plant J. 14: 475–481.
Seo, S., Okamoto, M., Ishizuka, K., Sano, H. and Ohashi Y. 1995. Tobacco MAP kinase: a possible mediator in wound signal transduction pathways. Science 270: 1988–1190.
Seo, S., Sano, H. and Ohashi, Y. 1999. Jasmonate-based wound signal transduction requires activation of WIPK, a tobacco mitogen-activated protein kinase. Plant Cell 11: 289–298.
Smirnov, S., Shulaev, V. and Tumer, N.E. 1997. Expression of pokeweed antiviral protein in transgenic plants induces virus resistance in grafted wild-type plants independently of salicylic acid accumulation and pathogenesis-related protein synthesis. Plant Physiol. 114: 1113–1121.
Stirpe, F., Barbieri, L., Battelli, M.G., Soria, M. and Lappi, D.A. 1992. Ribosome inactivating proteins from plants. Bio/tech. 10: 405–412.
Tomlinson, J.A., Walker, V.M., Flewett, T.H. and Barclay, G.R. 1974. The inhibition of infection by cucumber mosaic virus and influenza virus by extracts from Phytolacca americana. J. Gen. Virol. 22: 225–232.
Tumer, N.E., Hwang, D.-J. and Bonness, M. 1997. A nontoxic Cterminal deletion mutant of pokeweed antiviral protein inhibits viral infection. Proc. Natl. Acad. Sci. USA 94: 3866–2871.
Ussery, M.A., Irvin, J.D. and Hardesty, B. 1977. Inhibition of poliovirus replication by a plant antiviral peptide. Ann. N.Y. Acad. Sci. 284: 431–440.
vanWees, S.C.M., Pieterse, C.M.J., Trijssenaar, A., van'tWestende, Y.A.M., Hartog, F. and van Loon, L.C. 1997. Differential induction of systemic resistance in Arabidopsis by biocontrol bacteria. Mol. Plant-Microbe Interact. 10: 716–724.
Vidal, S, Ponce de Leon, I., Denecke, J. and Palva, E.T. 1997. Salicylic acid and the plant pathogen Erwinia carotovora induce defense genes via antagonistic pathways. Plant J. 11: 115–123.
Wang, P., Zoubenko, O. and Tumer, N.E. 1998. Reduced toxicity and broad-spectrum resistance to viral and fungal infection in transgenic plants expressing pokeweed antiviral protein II. Plant Mol. Biol. 38: 957–964.
Wyatt, S.D. and Shepherd, R.J. 1969. Isolation and characterization of virus inhibitor from Phytolacca americana. Phytopathology 59: 1787–1793.
Yalpani, N., Silverman, P., Wilson, T.M.A., Kieler, DA. and Raskin, I. 1991. Salicylic acid is a systemic signal and an inducer of pathogenesis-related proteins in virus infected tobacco. Plant Cell 3: 809–818.
Zarling, J.M., Moran, P.A., Haffar, O., Sias, J., Richman, D.D., Spina, C.A., Myers, D.E., Kuebelbeck, V., Ledbetter, J.A. and Uckun, F.M. 1990. Inhibition of HIV replication by pokeweed antiviral protein targeted to CD4C cells by monoclonal antibodies. Nature 347: 92–95.
Zhang, S. and Klessig, D.F. 1998. Resistance gene N-mediated de novo synthesis and activation of tobacco mitogen-activated protein kinase by tobacco mosaic virus infection. Proc. Natl. Acad. Sci. USA 95: 7433–7438.
Zoubenko, O., Uckun, F., Hur, Y., Chet, I. and Tumer, N.E. 1997. Plant resistance to fungal infection induced by nontoxic pokeweed antiviral protein mutants. Nature Biotechnol. 15: 992–996.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Zoubenko, O., Hudak, K. & Tumer, N.E. A non-toxic pokeweed antiviral protein mutant inhibits pathogen infection via a novel salicylic acid-independent pathway. Plant Mol Biol 44, 219–229 (2000). https://doi.org/10.1023/A:1006443626864
Issue Date:
DOI: https://doi.org/10.1023/A:1006443626864