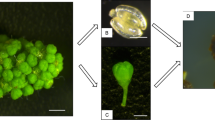
Abstract
Fourteen recombinant inbred lines of sunflower (Helianthus annuus L.) and their parents (PAC-2 and RHA-266) were tested for their organogenesis ability. Seeds were surface sterilized and germinated on hormone free half strength MS basal medium containing 10 g l-1 sucrose solidified with five different gelling agents: Phytagar (Gibco laboratoires) 3 g l-1, Phytagel (Sigma) 3 g l-1, Agarose (Sigma) 5 g l-1, Arcagel (Sigma) 4 g l-1 and Agar-Agar (Fisher France) 7 g l-1. Cotyledons from 2-day-old seedlings were split in half and the four explants of each seed were cultived in 55 mm diameter petri dishes containing 10 ml of MS medium supplemented with 50 μM KNO3, 1 μM myo-inositol, 5 μM casein hydrolysate, 4.4 μM of BA and 5.4 μM of NAA solidified with the same gelling agents. The experimental design was a randomized complete block with 3 replications. A replicate for each genotype consisted of ten petri dishes containing four explants. The statistical analysis showed significant differences among genotypes and gelling agents. Of the fourteen recombinant inbred lines tested `C93' presented the highest values for all regeneration traits in the five different media and it was better than the best parent. Agarose and Agar-Agar were more better than other gelling agents for shoot induction.
Similar content being viewed by others
References
Alibert G, Aslane-Chanabé CH & Burrus M (1994) Sunflower tissue and cell cultures and their use in biotechnology. Plant Cell. Biochem. 32: 31–44
Beruto M, Beruto D & Debergh P (1999) Influence of agar on in vitro. cultures: I. Physicochemical properties of agar and agar gelled media in vitro. Cell. Dev. Biol. Plant 35: 86–93
Bornam CH & Vogelman TC (1984) Effect of rigidity of gel medium on benzyladenine?induced adventitious bud formation and vitrification in vitro in Picea abies. Physiol. Plant. 61: 505–512
Brand MH (1993) Agar and ammonium nitrate influence hyperhydricity, tissue nitrate and total nitrogen content of serviveberry (Amelanchier arborea) shoots in vitro. Physiol. Plant. 61: 505–512
Chraibi BKM, Castelle JC, Latche A, Roustan JP & Fallot J (1992) Enhancement of shoot regeneration potential by liquid medium culture from nature cotyledons of sunflower (Helianthus annuus L.) Plant Cell. Rep. 10: 617–620
Debergh P, Harbaoui Y & Lemeur R (1981) Mass propagation of globe artichoke (Cynara scolymus): Evaluation of different hypotheses to overcome vitrification with special référence to water potential. Physiol Plant. 53: 181–187
Debergh PC (1983) Effects of agar brand and concentration on the tissue culture medium. Physiol. Plant 59: 270–276
Debergh P, Aitken?Christie J, Cohen D, Grout B, Von Arnold S, Zimmerman R & Zib M (1992) Reconsideration of the term 'vitrification' as used in micropropagation. Plant Cell. Tiss. Org. Cult. 40: 135–140
Deglene L, Lesignes P, Alibert G & Sarrafi A (1997), Genetic control of organogenesis in cotyledons of sunflower (Helianthus annuus L.). Plant Cell. Tiss. Org. Cult. 48: 127–130
Espinasse A, Lay C & Volin J (1989) Effects of growth regulator concentrations and explant size on shoot organogenesis from callus derived from zygotic embryos of sunflower (Helianthus annuus L.). Plant Cell. Tiss. Org. Cult. 17: 171–181
Ghaemi M & Sarrafi A (1993) Analysis of anther culture to measure genetic variability for embryogenesis in tetraploid wheat (Triticum turgidum var.). J. Genet. Breed. 47: 295–298
Kusumoto M (1980) Effects of coconut milk, agar and sucrose concentrations, and media pH on the proliferation of Cymbidium protocorm?like bodies cultured in vitro. J. Jap. Soc. Hortic. Sci. 48: 503–509
Lupi MC, Bennici A, Locci F & Gennai D (1987) Plantlet formation from callus and shoot tip culture of Helianthus annuus (L.) Plant Cell. Tiss. Org. Cult. 11: 47–55
Murashige T & Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant 15: 473–497
Nairn JB, Furneaux RH & Stevenson TT (1995) Identification of an agar constituent responsible for hydric control in micropropagation of radiata pine. Plant Cell Tiss. Org. Cult. 43: 1–11
Pâques M & Boxus PH (1987) 'Vitrification': review of literature. Acta Hortic. 212: 155–166
Pochet B, Scoman V, Mestdagh MM, Moreau B & Andre P (1991) Influence of agar gel properties on the in vitro micropropagation of different clones of Thuja plicata. Plant Cell. Rep 10: 406–409
Power CJ (1987) Organogenesis from Helianthus annuus inbreds and hybrids from the cotyledons of zygotic embryos An. J. Bot. 74: 497–503
Pugliesi C, Megale P, Cecconi F & Baroncelli S (1993) Organogenesis and embryogenesis in Helianthus tuberosus _ Helianthus tuberosus. Plant Cell. Tiss. Org. Cult. 33: 187–193
Quimio CA & Zapata FJ (1990) Diallel analysis of callus induction and green?plant regeneration in rice anther culture. Crop Sci. 30: 188–192
Reeds DA (1969) Structure, conformation and mecanism in the germination of polysaccharide gels and networks. Adv. Carbohydrate chem. Biochem. 24: 267–332
Sarrafi A, Bolandi AR, Serieys H, Berville A & Alibert G (1996a) Analysis of cotyledon culture to measure genetic variability for organogenesis parameters in sunflower (Helianthus annuus L.) Plant Science 121: 213–219
Sarrafi A, Roustan JP, Fallot J & Alibert G (1996b) Genetic analysis of organogenesis in the cotyledons of zygotic embryos of sunflower (Helianthus annuus L.). Theor. Appl. Genet. 92: 225–229
Singhas (1984) Influence of two commercial agars on in vitro shoot proliferation of 'Almey' Crabapple and 'Seckel' Pear. Hort. Sci. 19: 227–228
Wirtzens B, Scowcroft WR, Downes RW & Larkin PJ (1988) Tissue culture and plant regeneration from sunflower (Helianthus annuus) and interspecific hybrids (H. tuberosus _ H. annuus). Plant. Cell. Tiss. Org. Cult. 13: 61–76
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Berrios, E.F., Gentzbittel, L., Serieys, H. et al. Influence of genotype and gelling agents on in vitro regeneration by organogenesis in sunflower. Plant Cell, Tissue and Organ Culture 59, 65–69 (1999). https://doi.org/10.1023/A:1006433607812
Issue Date:
DOI: https://doi.org/10.1023/A:1006433607812