Abstract
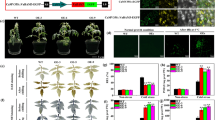
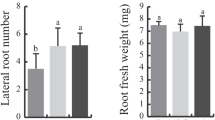
Pyruvate dehydrogenase kinase (PDHK), a negative regulator of the mitochondrial pyruvate dehydrogenase (PDH) complex (mtPDC), plays a pivotal role in controlling mtPDC activity, and hence, the TCA cycle and cell respiration. This report describes the cloning of a pyruvate dehydrogenase kinase cDNA (AtPDHK) from Arabidopsis thaliana and focuses on the effects of antisense down-regulation of its expression on plant growth and development. The deduced amino acid sequence of AtPDHK exhibits extensive similarity to other plant and mammalian PDHKs, containing conserved domains typical of two-component histidine protein kinases. The Escherichia coli expressed AtPDHK specifically phosphorylated mammalian PDH E1 in a time-dependent manner. Antisense expression of the AtPDHK cDNA led to marked elevation of mtPDC activity in transgenic plants with increases ranging from 137% to 330% compared to control plants. Immunoblot analyses performed with a monoclonal antibody to the E1α mtPDH component (the subunit phosphorylated by PDHK) indicated that the increased mtPDC activity was not the result of an increase in the level of PDH protein. MtPDC from transgenic plants showed a reduced sensitivity to ATP-dependent inactivation compared to that observed in wild-type plants. Collectively, these data suggest that the antisense partial silencing of the negative regulator, PDHK, was responsible for the increased mtPDC activity observed in the antisense PDHK plants. Transgenic plants with partially repressed AtPDHK also displayed altered vegetative growth with reduced accumulation of vegetative tissues, early flower development and shorter generation time. The potential role for AtPDHK gene manipulation in crop improvement is discussed.
Similar content being viewed by others
References
ap Rees, T., Bryce, J.H., Wilson, P.M. and Green, J.H. 1983. Role and location of NAD malic enzyme in thermogenic tissue of Araceae. Arch Biochem Biophys 227: 511–521.
Bechtold, N., Ellis. J. and Pelletier, G. 1993. In planta Agrobacterium-mediated gene transfer by infiltration of adult Arabidopsis thaliana plants. C.R. Acad. Sci. Ser. III Sci. Vie. 316: 1194–1199.
Bradford, M.M. 1976. A rapid and sensitive method for the quanti-tation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72: 248–254.
Budde, R.J.A. and Randall, D.D. 1990. Pea leaf mitochondrial pyruvate dehydrogenase complex is inactivated in vivo in a light-dependent manner. Proc. Natl. Acad. Sci. USA 87: 673–676.
Budde, R.J.A., Fang, T.K. and Randall, D.D. 1988. Regulation of the phosphorylation of mitochondrial pyruvate dehydrogenase complex in situ. Plant Physiol. 88: 1031–1036.
Coleman, J. 1990. Characterization of Escherichia coli cells defi-cient in 1-acyl-sn-glycerol-3-phosphate acyltransferase activity. J. Biol. Chem. 256: 17215–17221.
Coleman, J. 1992. Characterization of the Escherichia coli gene for 1-acyl-sn-glycerol-3-phosphate acyltransferase (plsC). Mol. Gen. Genet. 232: 295–303.
Datla, R.S.S., Hammerlindl, J.K., Panchuk, B., Pelcher, L.E. and Keller, W.A. 1992. Modified binary plant transformation vectors with the wild-type gene encoding NPTII. Gene 211: 383–384.
Datla, R.S.S., Bekkaoui, F., Hammerlindl, J., Pilate, G., Dun-stan, D.I. and Crosby, W.L. 1993. Improved high-level consti-tutive foreign gene expression in plants using an AMV RNA4 untranslated leader sequence. Plant Sci. 94: 139–149.
Elledge, S.J., Mulligan, J.T., Ramer, S.W., Spottswood, M. and Davis, R.W. 1991. _YES: a multifunctional cDNA expression vector for the isolation of genes by complementation of yeast and Escherichia coli mutations. Proc. Natl. Acad. Sci. USA 88: 1731–1735.
Grof, C.P.L., Winning, B.M., Scaysbrook, T.P., Hill, S.A. and Leaver, C.J. 1995. Mitochondrial pyruvate dehydrogenase: mole-cular cloning of the E1_ subunit and expression analysis. Plant Physiol. 108: 1623–1629.
Guan, Y., Rawsthorne, S., Scofield, G., Shaw, P. and Doonan, J. 1995. Cloning and characterization of a dihydrolipoamide acetyl-transferase (E2) subunit of the pyruvate dehydrogenase complex from Arabidopsis thaliana. J. Biol. Chem. 270: 5412–5417.
Havelange, A. 1980. The quantitative ultrastructure of the meris-tematic cells of Xanthium strumarium during the transition to flowering. Am. J. Bot. 67: 1171–1178.
Hill, R.L. and Bradshaw, R.A. 1969. Fumarase. Meth. Enzymol. 13: 91–99.
Hill, S.A., Grof, C.P.L., Bryce, J.H. and Leaver, C.J. 1992. Reg-ulation of mitochondrial function and biogenesis in cucumber (Cucumis sativus L.) cotyledons during early seedling growth. Plant Physiol. 99: 60–66.
Journet, E.P. and Douce, R. 1985. Enzymic capacities of purified cauliflower bud plastids for lipid synthesis and carbohydrate metabolism. Plant Physiol. 79: 458–467.
Katavic, V., Haughn, G.W., Reed, D., Martin, M. and Kunst, L. 1994. In planta transformation of Arabidopsis thaliana. Mol. Gen. Genet. 245: 363–370.
Katavic, V., Reed, D.W., Taylor, D.C., Giblin, E.M., Barton, D.L., Zou, J.-T., MacKenzie, S.L., Covello, P.S. and Kunst, L. 1995. Alteration of fatty acid composition by an EMS-induced mutation in Arabidopsis thaliana affecting diacylglycerol acyl-transferase activity. Plant Physiol 108: 399–409.
Knowles, V.L., McHugh, S.G., Hu, Z., Dennis, D.T., Miki, B. and Plaxton, W.C. 1998. Altered growth of transgenic tobacco lacking leaf cytosolic pyruvate kinase. Plant Physiol. 116: 45–51.
Korotchkina, L.G. and Patel, M.S. 1995. Mutagenesis studies of the phosphorylation sites of recombinant human pyruvate dehydrogenase. J. Biol. Chem. 270: 14297–14304.
Korotchkina, L.G., Tucker, M.M., Thekkumkara, T.J., Madhusud-han, K.T., Pons, G., Kim, H. and Patel, M.S. 1995. Over-expression and characterization of human tetrameric pyruvate. dehydrogenase and its individual subunits. Prot. Express. Purif. 6: 79–90.
Landschütze, V., Willmitzer, L. and Müller-Röber, B. 1995. Inhibi-tion of flower formation by antisense repression of mitochondrial citrate synthase in transgenic potato plants leads to a specific disintegration of the ovary tissues of flowers. EMBO J. 14: 660–666.
Lernmark, U. and Gardeström, P. 1994. Distribution of pyruvate dehydrogenase complex activities between chloroplasts and mi-tochondria from leaves of different species. Plant Physiol. 106: 1633–1638.
Lindstrom, J.T. and Vodkin, L.O. 1991. A soybean cell wall protein is affected by seed colour genotype. Plant Cell 3: 561–571.
Liu, S., Baker, J.C. and Roche, T.E. 1995. Binding of the pyru-vate dehydrogenase kinase to recombinant constructs containing the inner lipoyl domain of the dihydrolipoyl acetyltransferase component. J. Biol. Chem. 270: 793–800.
Luethy, M.H., Miernyk, J.A. and Randall, D.D. 1994. The nu-cleotide and deduced amino acid sequences of a cDNA encoding the E1 beta-subunit of the Arabidopsis thaliana mitochondr-ial pyruvate dehydrogenase complex. Biochim. Biophys. Acta. 1187: 95–98.
Luethy, M.H., Miernyk, J.A. and Randall, D.D. 1995. The mitochondrial pyruvate dehydrogenase complex: nucleotide and deduced amino-acid sequences of a cDNA encoding the Ara-bidopsis thaliana E1_-subunit. Gene 164: 251–254.
MacDonald, E.D. and ap Rees, T. 1983. Enzymic properties of amy-loplasts from suspension cultures of soybean. Biochim. Biophys. 755: 81–89.
Parkinson, J.S. and Kofoid, E.C. 1992. Communication modules in bacterial signaling proteins. Annu. Rev. Genet. 26: 71–112.
Pearson, W.R. and Lipman, D.J. 1988. Improved tools for bio-logical sequence comparison. Proc. Natl. Acad. Sci. USA 85: 2444–2448.
Popov, K.M., Zhao, Y., Shimomura, Y., Kuntz, M.J. and Har-ris, R.A. 1992. Branched-chain _-ketoacid dehydrogenase ki-nase: molecular cloning, expression and sequence similarity with histidine protein kinase. J. Biol. Chem. 267: 13127–13130.
Popov, K.M., Kedishvili, N.Y., Zhao, Y., Shimomura, Y., Crabb, D.W. and Harris, R.A. 1993. Primary structure of pyruvate dehy-drogenase kinase establishes a new family of eukaryotic protein kinases. J. Biol. Chem. 268: 26602–26606.
Popov, K.M., Kedishvili, N.Y., Zhao, Y., Gudi, R. and Harris, R.A. 1994. Molecular cloning of the p45 subunit of pyruvate dehydrogenase kinase J. Biol. Chem. 269:29720–29724.
Randall, D.D., Rubin, P.M. and Fenko, M. 1977. Plant pyruvate dehydrogenase complex purification, characterization and regulation by metabolites and phosphorylation. Biochim. Bio-phys. Acta 485: 336–349.
Randall, D.D., Williams, M. and Rapp, B.J. 1981. Phosphorylation-dephosphorylation of pyruvate dehydrogenase complex from pea leaf mitochondria. Arch. Biochem. Biophys. 207: 437–444.
Reid, E.E., Thompson, P., Lyttle, R. and Dennis, D.T. 1977. Pyru-vate dehydrogenase complex from higher plant mitochondria and proplastids. Plant Physiol. 59: 842–848.
Sambrook, J., Fritsch, E.F. and Maniatis, T. 1989. Molecular Cloning: A Laboratory Manual, 2nd ed., Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
Southern, E.M. 1975. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J. Mol. Biol. 98: 503–517.
Taylor, D.C., Magus, J.R., Bhella, R., Zou, J.-T., MacKenzie, S.L., Giblin, E.M., Pass, E.W. and Crosby, W.L. 1992. Biosynthesis of triacylglyerols in Brassica napus L. cv. Reston. Target: trierucin. In: S.L. MacKenzie and D.C. Taylor (Eds.), Seed Oils for the Future, AOCS Press, Champaign, IL, pp. 77–102.
Thelen, J.J., Muszynski, M.G., Miernyk, J.A. and Randall, D.D. 1998a. Molecular analysis of two pyruvate dehydrogenase ki-nases from maize. J. Biol. Chem. 273: 26618–26623.
Thelen, J.J., Miernyk, J.A. and Randall, D.D. 1998b. Nucleotide and deduced amino acid sequences of the pyruvate dehydrogenase kinase from Arabidopsis thaliana. Plant Physiol. 118: 1533.
Thelen, J.J., Miernyk, J.A. and Randall, D.D. 1999. Molecular cloning and expression analysis of the mitochondrial pyruvate dehydrogenase from maize. Plant Physiol. 119: 635–644.
Veeger, C., Der Vartanian, D.V. and Zeylemaker, W.P. 1969. Succinate dehydrogenase. Meth. Enzymol. 13: 81–90.
Winter, K., Foster, J.G., Edwards, G.E. and Holtum, J.A.M. 1982. Intracellular localization of enzymes of carbon metabolism in Mesembryanthemum crystallinum exhibiting C 3 photosynthetic characteristics or performing crassulacean acid metabolism. Plant Physiol. 69: 300–307.
Zhang, H., Goodman, H.M. Jansson, S. 1997. Antisense in-hibition of photosystem I antenna protein Lhca4 in Arabidopsis thaliana. Plant Physiol. 115: 1525–1531.
Zou, J.-T., Katavic, V., Giblin, E.M., Barton, D.L., MacKenzie, S.L., Keller, W.A., Hu, X. and Taylor, D.C. 1997. Modifica-tion of seed oil content and acyl composition in Brassicaceae by expression of a yeast sn-2 acyltransferase gene. Plant Cell 9: 909–923.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Zou, J., Qi, Q., Katavic, V. et al. Effects of antisense repression of an Arabidopsis thaliana pyruvate dehydrogenase kinase cDNA on plant development⋆. Plant Mol Biol 41, 837–849 (1999). https://doi.org/10.1023/A:1006393726018
Issue Date:
DOI: https://doi.org/10.1023/A:1006393726018