Abstract
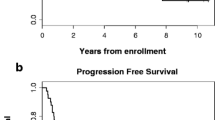
Background. Chemotherapeutic treatments containingtopoisomerase I inhibitors have shown antitumor activity against anumber of solid tumors. Responses have been seen in Phase I trialsusing topotecan in ovarian, lung, and esophageal cancer. A phase II trial using continuous infusion topotecan was completed toassess activity in esophagus cancer. Methods. Forty-fiveeligible patients with locally-advanced or metastatic squarnouscell carcinoma or adenocarcinoma of the esophagus received a regimen consisting of 24-hour continuous infusion topotecan at 1.5mg/m2/day on Days 1, 8, 15, 22 (of 42-day cycle). Patientscontinued on treatment until evidence of disease progression orunacceptable toxicity. Results. Partial response was demonstrated in 1 patient (2% confirmed response rate). Thirty-sixpatients progressed during the first cycle of treatment. The mediansurvival was 3 months, and the median progression-free survival was1 month. Toxicity was mild with only one Grade 4 toxicity reported.Conclusions. This phase II trial indicates nosignificant anti-neoplastic activity for topotecan administered inthe dose and schedule to patients with squamous cell oradenocarcinoma of the esophagus.
Similar content being viewed by others
References
Kingsbury WD, Herzrtberg RP, Boehm JC, Holden KG, Jakas DR, Caranta MJ, McCabe FL, Faucette LF, Johnson RK: Chemical synthesis and structure activity relationships related to SKF 104864, a novel water-soluble analog of camptothecin. (Abstract). Proc Am Assoc Cancer Res 30: 2475A, 1989
Hsiang Y-H, Herzbert R, Hecht S, Liu, LF: Camptothecin induces protein-linked DNA breaks via mammalian DNA topoisomerase I. J Biol Chem 260: 14873–14878, 1985
Burris H, Kuhn J, Johnson R, Von Hoff D: SKF: 104864: Preclinical studies of a new topoisomerase I inhibitor. (Abstract). Proc Amer Assoc Cancer Res 31: 2558A, 1990
Rowinsky E, Grochow L, Hendricks C, Sartorious S, Ettinger D, McGuire W, Forastiere A, Hurowitz L, Easter V, Downhower R: Phase I and pharmacological study of topotecan (SKF 104864): a novel topoisomerase I inhibitor. (Abstract). Proc Amer Soc Clin Oncol 10: 240A, 1991
Sirott MN, Saltz L, Young C, Tong W, Trochanowski B, Niedzwieki D, Toomasi F, Kelsen D: Phase I and clinical pharmacologic study of intravenous topotecan (T). (Abstract). Proc Amer Soc Clin Oncol 10: 284A, 1991
Wall J, Burris H, Rodriguez G, Brown T, Weiss G, Kuhn J, Brown J, Johnson R, Friedman C, Mann W, Von Hoff D: Phase I trial of topotecan (SKF 104684) in patients with refractory solid tumors. (Abstract). Proc Amer Soc Clin Oncol 10: 261A, 1991
Recondo G, Abbruzzese J, Newman B, Newman R, Kuhn J, von off D, Garteiz D, Raber M: A phase I trial of topotecan (TOPO) administered by 24-hour infusion. Proc Amer Assoc Cancer Res 32: 1229A, 1991
Kaplan EL, Meier P: Nonparametric estimation from incomplete observations. J Am Stat Assoc 53: 457–481, 1958
Enzinger P, Ilson DH, Saltz KB, O'Reilly EM, Gollub MJ, DeGroff JA, Nelson DP: A Phase II trial of cisplatinum and irinotecan in patients with advanced esophageal cancer. (Abstract). Proc Amer Soc Clin Oncol 17: 282A, 1998
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Macdonald, J.S., Jacobson, J.L., Ketchel, S.J. et al. A Phase II Trial of Topotecan in Esophageal Carcinoma: A Southwest Oncology Group Study (SWOG 9339). Invest New Drugs 18, 199–202 (2000). https://doi.org/10.1023/A:1006390216220
Issue Date:
DOI: https://doi.org/10.1023/A:1006390216220