Abstract
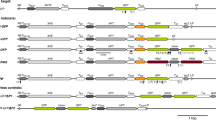
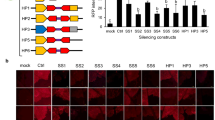
We have previously reported that the introduction of a full-length tobacco nitrite reductase Nii1 cDNA under the control of the 35S promoter triggers co-suppression of endogenous Nii genes in 25% of tobacco transformants. Here we show that introduction of chimeric Nii1-uidA, uidA-Nii1 and Nii1-uidA-Nii1 transgenes carrying 186 bp of the 5′ end and/or 241 bp of the 3′ end of the Nii1 cDNA do not trigger co-suppression of endogenous Nii genes. In addition, we show that when introduced by crossing or transformation into co-suppressed transgenic tobacco lines carrying full-length Nii1 transgenes, these chimeric transgenes are not silenced. These results therefore suggest that the 5′ and 3′ ends of the Nii1 cDNA are not sufficient to trigger co-suppression and are not targets for homology-dependent RNA degradation. Surprisingly, co-suppression was released in a double transformant obtained by introduction of one of these constructs into the co-suppressed transgenic tobacco line 461-2.1 homozygous for a full-length Nii1 transgene, and in one plant regenerated from untransformed leaf discs (plant 461-2.1*). The reappearance of co-suppression at very low frequency (less than 10−3) in the F2 progeny of plant 461-2.1* and the apparent absence of structural modification of the transgene locus suggest a metastable epigenetic modification. The steady-state level of Nii mRNAs in the plant 461-2-.1* was higher than in wild-type plants but lower than in hemizygous plants 461-2.1 which never trigger silencing. These results therefore confirm that transcription of the transgene above a particular threshold is required to trigger co-suppression.
Similar content being viewed by others
References
Bourgin, J.P., Chupeau, Y. and Missonier, C. 1979. Plant regeneration from mesophyll protoplasts of several Nicotiana species. Physiol. Plant. 45: 288-292.
Boutry, M. and Chua, N.M. 1985. A nuclear gene encoding the _ subunit of the mitochondrial ATP synthase in Nicotiana plumbaginifolia. EMBO J. 4: 2159-2165.
Bullock, W.O., Fernandez, J.M. and Short, J.M. 1987. XL1-Blue: a high efficiency plasmid transforming recA Escherichia coli strain with beta-galactosidase selection. BioTechniques 5: 376-378.
Coïc, Y. and Lesaint, C. 1971. Comment assurer une bonne nutrition en eau et ions minéraux en horticulture. Hort. Fr. 8: 11-14.
Crété, P., Caboche, M. and Meyer, C. 1997. Nitrite reductase expression is regulated at the post-transcriptional level by the nitrogen source in Nicotiana plumbaginifolia and Arabidopsis thaliana. Plant J. 11: 625-634.
de Caravalho, F., Gheysen, G., Kushnir, S., Van Montagu, M., Inzé, D. and Castresana, C. 1992. Suppression of _-1,3-glucanase transgene expression in homozygous plants. EMBO J. 11: 2595-2602.
Depicker, A. and Van Montagu, M. 1997. Post-transcriptional gene silencing in plants. Curr. Opin. Cell Biol. 9: 373-382.
Dorlhac de Borne, F., Vincentz, M., Chupeau, Y. and Vaucheret, H. 1994. Co-suppression of nitrate reductase host genes and transgenes in transgenic tobacco plants. Mol. Gen. Genet. 243: 613-621.
Dougherty, W.G. and Parks, T.D. 1995. Transgenes and gene suppression: telling us something new? Curr. Opin. Cell Biol. 7: 399-405.
Elmayan, T. and Vaucheret, H. 1996. Expression of single copies of a strongly expressed 35S transgene can be silenced posttranscriptionally. Plant J. 9: 787-797.
English, J.J. and Baulcombe, D.C. 1997. The influence of small changes in transgene transcription on homology-dependent virus resistance and gene silencing. Plant J. 12: 1311-1318.
English, J.J., Mueller, E. and Baulcombe, D.C. 1996. Suppression of virus accumulation in transgenic plants exhibiting silencing of nuclear genes. Plant Cell 8: 179-188.
Goodwin, J., Chapman, K., Swaney, S., Parks, T.D., Wernsman, E.A. and Dougherty, W.G. 1996. Genetic and biochemical dissection of transgenic RNA-mediated virus resistance. Plant Cell 8: 95-105.
Grierson, D., Fray, R.G., Hamilton, A.J., Smith, C.J.S. and Watson, C.F. 1991. Does co-suppression of sense genes in transgenic plants involve antisense RNA? Trends Biotechnol. 9: 122-123.
Gritz, L. and Davies, J. 1983. Plasmid-encoded hygromycin B resistance: the sequence of hygromycin B phosphotransferase gene and its expression in Escherichia coli and Saccharomyces cerevisiae. Gene 25: 179-188.
Hobbs, S.L.A., Kpodar, P. and Delong, C.M.O. 1993. Transgene copy number can be positively or negatively associated with transgene expression. Plant Mol. Biol. 21: 17-26.
Horsch, R.B., Fry, J.E., Hoffman, N.L., Eicholtz, D., Rogers, S.G. and Fraley, R.T. 1985. A simple and general method for transferring genes into plants. Science 227: 1229-1231.
Jefferson, R.A. 1987. Assaying chimeric genes in plants: the GUS gene fusion system. Plant Mol. Biol. Rep. 5: 387-405.
Jorgensen, R. 1992. Silencing of plant genes by homologous transgenes. AgBiotech. News Inf. 4: 265N-273N.
Jorgensen, R., Cluster, P.D., English, J., Que, Q. and Napoli, C. 1996. Chalcone synthase co-suppression phenotypes in petunia flowers: comparison of sense vs. antisense constructs and singlecopy vs. complex T-DNA sequences. Plant Mol. Biol. 31: 957-973.
Kronenberger, J., Lepingle, A., Caboche, M. and Vaucheret, H. 1993. Cloning and expression of distinct nitrite reductases in tobacco leaves and roots. Mol. Gen. Genet. 236: 203-208.
Lee, K.Y., Baden, C., Howie, W.J., Bedbrook, J. and Dunsmuir, P. 1997. Post-transcriptional gene silencing of ACC synthase in tomato results from cytoplasmic RNA degradation. Plant J. 12: 1127-1137.
Meins, F. Jr. and Kunz, C. 1994. Silencing of chitinase expression in transgenic plants: an autoregulatory model. In: J. Paszkowski (Ed.), Homologous Recombination and Gene Silencing in Plants, Kluwer Academic Publishers, Dordrecht, Netherlands, pp. 335-348.
Metzlaff, M., O'Dell, M., Cluster, P.D. and Flavell, R.B. 1997. RNA-mediated RNA degradation and chalcone synthase A silencing in Petunia. Cell 88: 1-20.
Meyer, P. 1995. Gene Silencing in Higher Plants and Related Phenomena in Other Eukaryotes, Springer-Verlag, Berlin/Heidelberg.
Meyer, P., Heidmann, I. and Niedenhof, I. 1993. Differences in DNA-methylation are associated with a paramutation phenomenon in transgenic petunia. Plant J. 4: 89-100.
Neuhuber, F., Park, Y.D., Matzke, A.J.M. and Matzke, M.A. 1994. Susceptibility of transgene loci to homology-dependent gene silencing. Mol. Gen. Genet. 244: 230-241.
Palauqui, J.-C., Elmayan, T., Dorlhac de Borne, F., Crété, P., Charles, C. and Vaucheret, H. 1996. Frequencies, timing, and spatial patterns of co-suppression of nitratre reductase and nitrite reductase in transgenic tobacco plants. Plant Physiol. 112: 1447-1456.
Pang, S.Z., Jan, F.J. and Gonsalves, D. 1997. Nontarget DNA sequences reduce the transgene length necessary for RNAmediated tospovirus resistance in transgenic plants. Proc. Natl. Acad. Sci. USA 94: 8261-8266.
Park, Y.D., Papp, I., Moscone, E.A., Iglesias, V.A., Vaucheret, H., Matzke, A.J.M. and Matzke, M.A. 1996. Gene silencing mediated by promoter homology occurs at the level of transcription and results in meiotically heritable alterations in methylation and gene activity. Plant J. 9: 183-194.
Schiebel, W., Pélissier, T., Riedel, L., Thalmeir, S., Schiebel, R., Kempe, D., Lottspeich, F., Sänger, H.L. and Wasseneger, M. 1998. Isolation of an RNA-directed RNA polymerase-specific cDNA clone from tomato. Plant Cell 10: 2087-2101.
Seymour, G.B., Fray, R.G., Hill, P. and Tucker, G.A. 1993. Downregulation of two non-homologous endogenous tomato genes with a single chimeric sense gene construct. Plant Mol. Biol. 23: 1-9.
Sijen, T., Wellinck, J., Hiriart, J.B. and van Kammen, A. 1996. RNA-mediated virus resistance: role of repeated transgenes and delineation of targeted regions. Plant Cell 8: 2277-2294.
Stam, M., Mol, J.N.M. and Kooter, J.M. 1997. The silence of genes in transgenic plants. Ann. Bot. 79: 3-12.
Tanzer, M.M., Thompson, W.F., Law, M.D., Wernsman, E.A. and Uknes, S. 1997. Characterization of post-transcriptionally suppressed transgene expression that confers resistance to tobacco etch virus infection in tobacco. Plant Cell 9: 1411-1423.
Topfer, R., Matzeit, V., Gronenborn, B., Schell, J. and Steinbiss, H.H. 1987. A set of plant expression vectors for transcriptional and translational fusions. Nucl. Acids Res. 14: 5890.
van Blokland, R., Vandergeest, N., Mol, J.N.M. and Kooter, J.M. 1994. Transgene-mediated suppression of chalcone synthase expression in Petunia hybrida results from an increase in RNA turnover. Plant J. 6: 861-877.
Vaucheret, H. 1993. Identification of a general silencer for 19S and 35S promoters in a transgenic tobacco plant-90 bp of homology in the promoter sequence are sufficient for trans-inactivation. C.R.A. Acad. Paris Sci. III 316: 1471-1483.
Vaucheret, H., Palauqui, J.C., Elmayan, T. and Moffatt, B. 1995. Molecular and genetic analysis of nitrite reductase cosuppression in trangenic tobacco plants. Mol. Gen. Genet. 248: 311-317.
Voinnet, O., Vain, P., Angell, S. and Baulcombe, D.C. 1998. Systemic spread of sequence-specific transgene RNA degradation in plants is initiated by localized introduction of ectopic promoterless DNA. Cell 95: 177-187.
Wasseneger, M. and Pélissier, T. 1998. A model for RNA-mediated gene silencing in higher plants. Plant Mol. Biol. 37: 349-362.
Wasseneer, M., Heimes, S., Riedel, L. and Sänger, H.L. 1994. RNAdirected de novo methylation of genomic sequences in plants. Cell 76: 567-576.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Crété, P., Vaucheret, H. Expression and sequence requirements for nitrite reductase co–suppression. Plant Mol Biol 41, 105–114 (1999). https://doi.org/10.1023/A:1006364323494
Issue Date:
DOI: https://doi.org/10.1023/A:1006364323494