Abstract
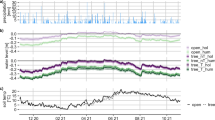
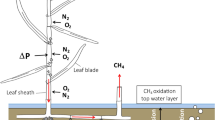
The aim of this study was to correlate magnitude andcontrols of CH4 fluxes with the microtopographyand the vegetation in a hollow-ridge complex of araised bog. High CH4 emission rates were measuredfrom hollows and mud-bottom hollows, while hummocksconsumed atmospheric CH4 at a low rate. Thehighest emissions were measured from plots with Eriophorum vaginatum and Scheuchzeriapalustris. CH4 emission ceased after Scheuchzeria had been clipped below the water table,indicating the importance of this aerenchymatic plantas a conduit for CH4.
Peat in the upper catotelm of hollows was younger andless decomposed than in hummocks. Potential CH4production in vitro was higher and themethanogenic association was better adapted to highertemperatures in hollow than in hummock peat. Highertemperatures in hollows resulted in a strongerCH4 source in hollows than in hummocks. Negativefluxes from hummocks indicated that even in wetlandsmethanotrophic bacteria exist that are able to oxidizeCH4 at atmospheric mixing ratios, and thatoxidation controls CH4 emission completely. TheCH4 mixing ratio was low in the acrotelm, but itincreased within the catotelm. Comparing fluxesmeasured in static chambers with fluxes calculatedfrom the porewater CH4 profiles it was deducedthat the zone of methane oxidation was located closeto the water table.
In hollows, CH4 production at in situtemperature was far higher than emission into theatmosphere, corresponding to an oxidation rate ofnearly 99%. The CH4 flux between the catotelmand the acrotelm of hollows was also higher than theemission, indicating the importance of CH4oxidation in the aerobic acrotelm, too. CH4microprofiles showed that CH4 oxidation inmud-bottom hollows was confined to the topmost 2 mm,and that in Sphagnum-covered hollows CH4oxidation occurred at the lower edge of green Sphagnum-parts.
Similar content being viewed by others
References
Armstrong W(1964) Oxygen diffusion from the roots of some British bog plants. Nature 204: 801–802
Barber DA, Ebert M & Evans NTS (1962) The movement of 15O through barley and rice plants. J. Exper. Botany 13: 397–403
Bartlett KB & Harriss RC (1993) Review and assessment of methane emissions fromwetlands. Chemosphere 26: 261–320
Benstead J & Lloyd D (1996) Spatial and temporal variations of dissolved gases (CH4, CO2, and O2) in peat cores. Microb. Ecol. 31: 57–66
Bosse U & Frenzel P (1998) Methane emissions from rice microcosms: The balance of production, accumulation and oxidation. Biogeochemistry 41: 199–214
Bubier JL, Moore TR, Bellisario L, Comer NT & Crill PM (1995) Ecological controls on methane emissions from a northern peatland complex in the zone of discontinuous permafrost, Manitoba, Canada. Global Biogeochem. Cycles 9: 455–470
Cao M, Gregson K & Marshall S (1998) Global methane emission from wetlands and its sensitivity to climate change. Atmos. Environ. 32: 3293–3299
Chanton JP, Shrakey JWH & Dacey TD (1991) Effects of vegetation on methane flux, reservoirs, and carbon isotopic composition. In: Holland EA & Mooney HA (Eds) Trace Gas Emission from Plants (pp 65–92). Academic Press, San Diego
Chapman SJ & Thurlow M (1996) The influence of climate on CO2 and CH4 emissions from organic soils. Agricult. Forest Meteorol. 79: 205–217
Christensen TR (1993) Methane emission from arctic tundra. Biogeochemistry 21: 117–139
Christensen TR & Cox P (1995) Response of methane emission from arctic tundra to climatic change - results from a model simulation. Tellus Ser. B-Chem. Phys. Meteorol. 47: 301–309
Denier van der Gon HAC & Neue HU (1996) Oxidation of methane in the rhizosphere of rice plants. Biol. Fertil. Soils 22: 359–366
Dunfield P, Knowles R, Dumont R & Moore TR (1993) Methane production and consumption in temperate and subarctic peat soils - response to temperature and pH. Soil Biol. Biochem. 25: 321–326
Fechner EJ & Hemond HF (1992) Methane transport and oxidation in the unsaturated zone of a Sphagnum peatland. Global Biogeochem. Cycles 6: 33–44
Frenzel P (2000) Plant-associated methane oxidation in ricefields and wetlands. Adv. Microb. Ecol. 16: in press
Frenzel P & Bosse U (1996) Methyl fluoride, an inhibitor of methane oxidation and methane production. FEMS Microbiol. Ecol. 21: 25–36
Frenzel P & Rudolph J (1998) Methane emission from a wetland plant: the role of CH4 oxidation in Eriophorum. Plant Soil 202: 27–32
Frenzel P, Thebrath B & Conrad R (1990) Oxidation of methane in the oxic surface layer of a deep lake sediment (Lake Constance). FEMS Microbiol. Ecol. 73: 149–158
Gilbert B & Frenzel P (1995) Methanotrophic bacteria in the rhizosphere of rice microcosms and their effect on porewater methane concentration and methane emission. Biol. Fert. Soils 20: 93–100
Gore AJP (1983) Ecosystems of the World 4A. Mires: Swamp, Bog, Fen and Moor. General Studies. Elsevier Scientific Publishing Company, Amsterdam
Heyer J & Suckow R (1985) Ökologische Untersuchungen der Methanoxidation in einem sauren Moorsee. Limnologica (Berlin) 16: 247–266
Holzapfel-Pschorn A, Conrad R & Seiler W (1985) Production, oxidation and emission of methane in rice paddies. FEMS Microbiol. Ecol. 31: 343–351
Hulme PD (1986) The origin and development of wet hollows and pools on Graigeazle Mire, South-West Scotland. Int. Peat J. 1: 15–28
Hyman MR & Arp DJ (1987) Quantification and removal of some contaminating gases from acetylene used to study gas-utilizing enzymes and microorganisms. Appl. Environ. Microbiol. 53: 298–303
Ilomets M (1979) Dynamics of fractional composition of peat forming layer on the Männikjärve bog (in Russian). In: Raukas A (Ed) Physical, Isotope-geochemical and Geological Methods in Quaternary Studies of Estonia (pp 22–26). Academy of Estonian S.S.R. Publishers
Tallinn Ilomets M (1982) The productivity of Sphagnum communities and the rate of peat accumulation in Estonian bogs. In: Masing V (Ed) Peatland Ecosystems (pp 102–116). Academy of Sciences of the Estonian S.S.R.
Tallinn Ingram HAP (1978) Soil layers in mires: Function and terminology. J. Soil. Science 29: 224–227
Iversen N & Joergensen BB (1985) Anaerobic methane oxidation rates at the sulfatemethane transition in marine sediments from Kattegat and Skagerrak (Denmark). Limnol. Oceanogr. 30: 944–955
Janssen PH & Frenzel P (1997) Inhibition of methanogenesis by methyl fluoride: Studies on pure and defined mixed cultures of anaerobic bacteria and archaea. Appl. Environ. Microbiol. 63: 4552–4557
Kiene RP (1991) Production and consumption of methane in aquatic systems. In: Rogers JE & Whitman WB (Ed) Microbial Production and Consumption of Greenhouse Gases: Methane, Nitrogen Oxides, and Halomethanes (pp 111–146). American Society for Microbiology, Washington, D.C.
King GM (1990) Dynamics and controls of methane oxidation in a Danish wetland sediment. FEMS Microbiol. Ecol. 74: 309–324
King GM, Roslev P & Skovgaard H (1990) Distribution and rate of methane oxidation in sediments of the Florida Everglades. Appl. Environ. Microbiol. 56: 2902–2911
King JY, Reeburgh WS & Regli SK (1998) Methane emission and transport by arctic sedges in Alaska - results of a vegetation removal experiment. J. Geophys. Res.-Atmos. 103: 29,083–29,092
Krumholz LR, Hollenback JL, Roskes SJ & Ringelberg DB (1995) Methanogenesis and methanotrophy within a Sphagnum peatland. FEMS Microbiology Ecology 18: 215–224
Kuivila KM, Murray JW, Devol AH, Lidstrom ME & Reimers CE (1988) Methane cycling in the sediments of Lake Washington. Limnol. Oceanogr. 33: 571–581
Moosavi SC & Crill PM (1998) CH4 oxidation by tundra wetlands as measured by a selective inhibitor technique. J. Geophys. Res.-Atmos. 103: 29,093–29,106
Lehmann-Richter S, Großkopf R, Liesack W, Frenzel P & Conrad R (1999) Methanogenic archaea and CO2–dependent methanogenesis on washed rice roots. Environ. Microbiol. 1: 159–166
Lelieveld J, Crutzen PJ & Dentener FJ (1998) Changing concentration, lifetime and climate forcing of atmospheric methane. Tellus Ser. B-Chem. Phys. Meteorol. 50: 128–150
Lien T, Martikanen P, Nykanen H & Bakken L (1992) Methane oxidation and methane fluxes in two drained peat soils. Suo 43: 231–236
Matthews E & Fung I (1987) Methane emission from natural wetlands: global distribution, area, and environmental characteristics of sources. Global Biogeochem. Cycles 1: 61–86
Moore TR & Dalva M (1997) Methane and carbon dioxide exchange potentials of peat soils in aerobic and anaerobic laboratory incubations. Soil Biol. Biochem. 29: 1157–1164
Muller KL, Ganf GG & Boon PI (1994) Methane flux from beds of Baumea arthrophylla (Nees) Boeckeler and Triglochin procerum R. Br. at Bool Lagoon, South Australia. Austr. J. Marine Freshw. Res. 45: 1543–1553
Nedwell DB & Watson A (1995) CH4 production, oxidation and emission in a UK ombrotrophic peat bog: Influence of SO4 2–from acid rain. Soil Biol. Biochem. 27: 893–903
Oremland RS & Taylor BF (1975) Inhibition of methanogenesis in marine sediments by acetylene and ethylene: validity of the acetylene reduction assay for anaerobic microcosms. Appl. Environ. Microbiol. 30: 707–709
Prior SD & Dalton H (1985) Acetylene as a suicide substrate and active site probe for monooxygenase from Methylococcus capsulatus (Bath). FEMS Microbiol. Lett. 29: 105–109
Rothfuss F, Frenzel P & Conrad R (1994) Gas diffusion probe for measurement of CH4 gradients. In: Stal LJ & Caumette P (Ed)Microbial Mats (pp 167–172). NATO ASI Series, Vol. G 35
Rothfuss F, Bijnen FGC, Conrad R, Harren FJM & Reuss J (1996) Combination of photoacoustic detector with gas diffusion probes for the measurement of methane concentration gradients in submerged paddy soil. Chemosphere 33: 2487–2504
Saarnio S, Alm J, Silvola J, Lohila A, Nykanen H & Martikainen PJ (1997) Seasonal variation in CH4 emissions and production and oxidation potentials at microsites on an oligotrophic pine fen. Oecologia 110: 414–422
Schimel JP (1995) Plant transport and methane production as controls on methane flux from arctic wet meadow tundra. Biogeochemistry 28: 183–200
Sprott GD, Jarrell KF, Shaw KM & Knowles R (1982) Acetylene as an inhibitor of methanogenic bacteria. J. Gen. Microbiol. 128: 2453–2462
Stephen KD, Arah JRM, Thomas KL, Benstead J & Lloyd D (1998) Gas diffusion coefficient profile in peat determined by modelling mass spectrometric data - implications for gas phase distribution. Soil Biol. Biochem. 30: 429–431
Sundh I, Nilsson M & Svensson BH (1992) Depth distribution of methane production and oxidation in a sphagnum peat bog. Suo 43: 267–269
Sundh I, Mikkelä C, Nilsson M & Svensson BH (1995) Potential aerobic methane oxidation in a Sphagnum-dominated peatland - controlling factors and relation to methane emission. Soil Biol. Biochem. 27: 829–837
Updegraff K, Pastor J, Bridgham SD & Johnston CA (1995) Environmental and substrate controls over carbon and nitrogen mineralization in northern wetlands. Ecol. Applic. 5: 151–163
Uzaki M, Mizutani H & Wada E (1991) Carbon isotope composition of CH4 from rice paddies in Japan. Biogeochemistry 13: 159–175
Veber K (1974) Vegetational history of the Endla mire system. In: Kumari E (Ed) Estonian Wetlands and Their Life (pp 160–182). Academy of Sciences of the Estonian S.S.R., Estonian Committee for the IBP
Waddington JM & Roulet NT (1996) Atmosphere-wetland carbon exchanges: Scale dependency of CO2 and CH4 exchange on the developmental topography of a peatland. Global Biogeochem. Cycles 10: 233–245
Waddington JM, Roulet NT & Swanson RV (1996) Water table control of CH4 emission enhancement by vascular plants in boreal peatlands. J. Geophys. Res. 101: 22,775–22,785
Walter BP, Heimann M, Shannon RD & White JR (1996) A process-based model to derive methane emissions from natural wetlands. Geophys. Res. Lett. 23: 3731–3734
Watson A, Stephen KD, Nedwell DB & Arah JRM (1997) Oxidation of methane in peat - kinetics of CH4 and O2 removal and the role of plant roots. Soil Biol. Biochem. 29: 1257–1267
Whiting GJ & Chanton JP (1992) Plant-dependent CH4 emission in a subarctic Canadian fen. Global Biogeochem. Cycles 6: 225–231
Yavitt JB, Lang GE & Downey DM (1988) Potential methane production and methane oxidation rates in peatland ecosytems of the Appalachian Mountains, United States. Global Biogeochem. Cycles 2: 253–268
Yavitt JB, Downey DM, Lancaster E & Lang GE (1990) Methane consumption in decomposing Sphagnum-derived peat. Soil Biol. Biochem. 22: 441–447
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Frenzel, P., Karofeld, E. CH4 emission from a hollow-ridge complex in a raised bog: The role of CH4 production and oxidation. Biogeochemistry 51, 91–112 (2000). https://doi.org/10.1023/A:1006351118347
Issue Date:
DOI: https://doi.org/10.1023/A:1006351118347