Abstract
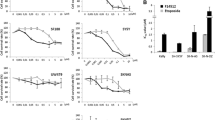
The efficacy of the epipodophyllotoxins VP-16 and VM-26 is limited by the occurrence of drug resistance in the tumor cell population. Cellular insensitivity to drugs that stabilize the cleavable complex is frequently expressed as multidrug resistance (MDR). In some cell lines, overexpression of MDR-1/P-glycoprotein or the multidrug resistance associated protein (MRP) has been demonstrated and implicated as the mechanism of resistance. Typically, these cells have reduced drug accumulation, secondary to increased drug efflux. In other cell lines, an atypical MDR phenotype has been identified, with the predominant mechanism of resistance shown to be qualitative and/or quantitative changes in the levels and activity of topoisomerase II. For VP-16, increased expression of MDR-1 or MRP and alterations in topoisomerase II α have been shown to confer tolerance. To further understand resistance to VP-16, T98G-VP(1000) was initially isolated as a single clone from parental cell, T98G, by exposure to VP-16. Subsequently, a population of cells from this subline was exposed to three-fold higher drug concentration allowing stable sublines to be established at higher extracellular drug concentration. Characterization of the resistant sublines demonstrates the adaptation that occurs with advancing drug concentrations during in vitro selections. Reduced topoisomerase II mRNA and protein levels were observed in the initial isolate. This reduction was accompanied by a decrease in topoisomerase II activity and cellular growth rate and was associated with 47-fold resistance to topoisomerase II poisons. With advancing resistance, MRP expression increased, with increased VP-16 efflux and reduced accumulation. This adaptation allowed for partial restoration of topoisomerase II activity secondary to increased expression and hyperphosphorylation, with a resultant increase in growth rate. In this cell line, hyperphosphorylation coincided with increased casein kinase II mRNA protein levels, without increased PKC protein levels, suggesting a role for this kinase in the acquired hyperphosphorylation. In this cell line, hyperphosphorylation mediated the increased activity despite a fall in topoisomerase IIα protein levels secondary to an acquired 615 bp deletion in one topoisomerase IIα allele, which resulted in reduced protein levels. In this subline, high levels of resistance were attained as a result of synergism between the reduced topoisomerase IIα levels and MRP overexpression. These studies demonstrate how cellular adaptation to increasing drug pressure occurs and how more than one mechanism can contribute to the resistant phenotype when increasing selecting pressure is applied. Reduced expression of topoisomerase II is sufficient to confer substantial resistance early in the selection process, with synergy from additional mechanisms helping to confer high levels of resistance.
Similar content being viewed by others
References
Beck WT, Kim R, Chen M: Novel actions of inhibitors of DNA topoisomerase II in drug-resistant tumor cells. Cancer Chemother Pharmacol 34(Suppl): S14–S18, 1994
Fojo AT, Ueda K, Slamon DJ, Poplack DG, Gottesman MM, Pastan I: Expression of a multidrug resistance gene in human tumors and tissues. Proc Natl Acad Sci USA 84: 265–269, 1987
Cole SPC, Bhardwaj G, Gerlach JH, Mackie JE, Grant CE, Almquist KC, Stewart AJ, Kurz EU, Duncan AMV, Deeley RG: Overexpression of a transporter gene in a multidrug resistant human lung cancer cell line. Science 258: 1650–1654, 1992
Cole SPC, Sparks KE, Fraser K, Loe DW, Grant CE, Wilson GM: Pharmacological characterization of multidrug resistant MRP-transfected human tumor cells. Cancer Res 54: 5902–5910, 1994
Grant CE, Vadimarsson G, Hipfner DR, Almquist KC, Cole SPC, Deeley RG: Overexpression of multidrug resistance associated protein increases resistance to natural product drugs. Cancer Res 54: 357–361, 1994
Lorico A, Rappa G, Srimatkandada S, Catapano CV, Fernandes DJ, Germino JF, Sartorelli AC: Increased rate of VP-16 efflux in a murine leukemia cell line overexpressing the multidrug associated protein gene. Cancer Res 55: 4352–4360, 1995
Matsumoto Y, Fujiwara T, Honjo Y, Sasaoka N, Tsuchi da T, Nagao S: Quantitative analysis of DNA topoisomerase I activity in human and rat glioma: characterization and mechanism of resistance to antitopoisomerase chemical, camptothecin-11. Brain Tumor Pathol 11: 59–64, 1994
Kaufmann SH, Karp JE, Jones RJ, Miller CB, Schneider E, Zwelling LA, Cowan K, Wendel K, Burke PJ: Topoisomerase II levels and drug sensitivity in adult acte myelogenous leukemia. Blood 83: 517–530, 1994
Takano H, Kohno K, Ono M, Ushida Y, Kuwano M: Increased phosphorylation of DNA topoisomerase II in etoposide resistant mutants of human cancer KB cells. Cancer Res 51: 3951–3957, 1991
Cardenas ME, Dang Q, Glover CVC, Gasser SM. Casein kinase II phosphorylates the eukaryote-specific c-terminal domain of topoisomerase II in vitro. EMBO J 11: 1785–1796, 1992
Wells NJ, Fry AM, Guano F, Norbury C, Hickson ID: Cell cycle phase-specific phosphorylatioin of human topoisomerase II?. J Biol Chem 269: 28357–28363, 1995
Alghisi GC, Roberts E, Cardenas ME, Gasser SM: The regulation of DNA Topoisomerase II by Casein Kinase II. Cell Mol Biol Res 40: 563–571, 1994
Caron PR, Wang JC: DNA topoisomerase as target of therapeutics: a structural overview. In: Andoh T, Ikade H, Oguro M(eds). Molecular Biology ofDNATopoisomerases and its Application to Chemotherapy. CRC Press Inc, Boca Raton, 1993, pp 2–18
Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, Warren JT, Bokesch H, Kenney S, Boyd MR: New colorimetric cytotoxicty assay for anticancer-drug screening. J Natl Cancer Inst 82: 1107–1112, 1990
Melton DA, Krieg PA, Rebagliati MR, Maniatis T, Zinn K, Green MR: Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res 12: 7035–7056, 1984
Mickley LA, Bates SE, Richert ND, Currier S, Tanaka S, Foss F, Rosen N, Fojo AT: Modulation of the expression of a multidrug resistance gene (mdr-1/P-glycoprotein) by differentiating agents. J Biol Chem 264: 18031–18040, 1989
Matsumoto Y, Fujiwara T, Honjo Y, Sasaoka N, Tsuchida T, Nagao S: Quantitative analysis of DNA topoisomerase I activity in human and rat glioma: characterization and mechanism of resistance to antitopoisomerase chemical, camptothecin-11. J Surg Oncol 53: 97–103, 1993
Hinds M, Deisseroth K, Mayes J, Altschuler E, Jansen R, Ledley FD, Zwelling LA: Identification of a point in the topoisomerase II gene from a human leukemia cell line containing an amsacrine-resistant form of topoisomerase II. Cancer Res 51: 4729–4731, 1991
Berger JC, Gamblin SJ, Harrison SC, Wang JC: Structure and mechanism of DNA topoisomerase II. Nature 379: 225–232, 1996
Vassetzky YS, Dang QI, Benedetiti P, Gasser SM: Topoisomerase II forms multimer in vitro: effects of metals, ?-glycerophosphate, and phosphorylation of its c-terminal domain. Mol Cell Biol 14: 6962–6974, 1994
Wells NJ, Addison CM, Fry AM, Ganapathi R, Hickson ID: Serine 1524 is a major site of phosphorylation on human topoisomerase IIa protein in vivo and is a substrate for casein kinase II in vitro. J Biol Chem 270: 29746–29751, 1994
Watt PM, Hickson ID: Structure and function of type IIDNA topoisomerases. Biochem J 303: 681–695, 1994
Shiozaki K, Yanagida M: Functional dissection of the phosphorylated termini of fission yeast DNA topoisomerase II. J Cell Biol 119: 1023–1036, 1992
Cole SPC, Chanda ER, Dicke FP, Gerlach JH, Mirski SEL: Non-P-glycoprotein-mediated multidrug resistance in a small cell lung cancer cell line: evidence for decreased susceptibility to drug induced DNA damage and reduced levels of topoisomerase II. Cancer Res 51: 3345–3352, 1991
Matsumoto Y, Morisaki K, Kunishio K, Nagao S, Takano H, Fojo T: Incidence of mutation and deletion in topoisomerase II mRNA of etoposide and m-AMSA resistant cell lines. Jpn J Cancer Chemother 25: 1145–1149, 1998
Matsumoto Y, Takano H, Nagao S, Iglesias A, Fojo T: Expression of DNA topoisomerases (I, II alpha, II beta) mRNA in etoposide-and mAMSA-resistant cell lines. Jpn J Cancer Chemother 24: 2265–2269, 1997
Matsumoto Y, Fujiwara T, Nagao S: Determinants of drug response in camptothechin-11-resistant glioma cell lines. J Neuro-Oncol 23: 1–8, 1995
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Matsumoto, Y., Kunishio, K. & Nagao, S. Increased Phosphorylation of DNA Topoisomerase II in Etoposide Resistant Mutants of Human Glioma Cell Line. J Neurooncol 45, 37–46 (1999). https://doi.org/10.1023/A:1006346624083
Issue Date:
DOI: https://doi.org/10.1023/A:1006346624083