Abstract
We assessed the peripheral neuropathic changes induced by biweekly combination chemotherapy including paclitaxel 100–165 mg/m2 (in a 3-h infusion), epirubicin 75 mg/m2 and cisplatin 50 mg/m2 (TEC) in patients with advanced ovarian cancer.
Neurologic evaluation, including a standardized questionnaire, bed-side neurological examination, and quantitative determination of vibratory perception thresholds (VPT) and grip strength took place before therapy, after 3 and 6 cycles, and thereafter whenever possible. During chemotherapy all patients received granulocyte colony-stimulating factor from days 2 to 12. Pretreated patients received amifostine two times, before epirubicin and before cisplatin administration.
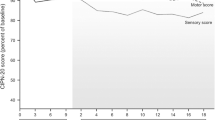
Neuropathic symptoms developed in 11/13 non-pretreated patients and in 7/9 chemotherapy-pretreated patients. Neuropathic signs developed in all patients. Neuropathic symptoms and signs were predominantly sensory in character. VPT changes developed primarily in the feet. According to National Cancer Institute of Canada Common Toxicity Criteria, grade 3 peripheral neuropathy after 6 cycles developed in 1/6 and 2/4 non-pretreated patients who received TEC containing paclitaxel 150 and 165 mg/m2, respectively.
We conclude that peripheral neuropathy is dose-limiting in chemonaïve patients treated with biweekly TEC combination chemotherapy, at paclitaxel dose level 165 mg/m2 in a 3-h intravenous administration.
Similar content being viewed by others
References
Vermorken JB, Pecorelli S: Clinical trials in patients with epithelial ovarian cancer: past, present and future. Eur J Surg Oncol 22: 455–466, 1996
Advanced Ovarian Cancer Trialists Group: Chemotherapy in advanced ovarian cancer: an overviewof randomised clinical trials. BMJ 303: 884–893, 1991
Neijt JP, ten Bokkel Huinink WW, van der Burg MEL, van Oosterom AT, Willemse PHB, Vermorken JB, van Lindert ACM, Heintz APM, Aartsen E, van Lent M, Trimbos JB, de Meijer AJ: Long term survival in ovarian cancer. Mature data from The Netherlands joint study group for ovarian cancer. Eur J Cancer 27: 1367–1372, 1991
Markman M, Bundy B, Benda J, Alberts D, Wadler S, Fowler J, Clark-Pearson D, Carson LF: Randomized phase 3 study of intravenous cisplatin/paclitaxel versus moderately high dose iv carboplatin followed by iv paclitaxel and intraperitoneal cisplatin in optimal residual ovarian cancer: an intergroup trial. Proc Am Soc Clin Oncol 17: 361a, 1998 (Abstract)
Alberts DS, LiuPY, HanniganEW, O'Toole R, Williams SD, Young JA, Franklin EW, Clarke-Pearson DL, Malviya VK, DuBeshter B, Adelson MD, Hoskins WJ: Intraperitoneal cisplatin plus intravenous cyclophosphamide versus intravenous cisplatin plus intravenous cyclophosphamide for stage III ovarian cancer.NEngl JMed 335: 1950–1955, 1996
McGuireWP, Rowinsky EK, Rosenshein NB, Grumbine FC, Ettinger DS, Armstrong DK, Donehower RC: Taxol: a unique antineoplastic agent with significant activity in advanced ovarian epithelial neoplasms.Ann Intern Med111: 273–279, 1989
Trimble EL, Adams JD, Vena D, Hawkins MJ, Friedman MA, Fisherman JS, Christian MC, Canetta R, Onetto N, Hayn R, Arbuck SG: Paclitaxel for platinumrefractory ovarian cancer: results from the first 1000 patients registered to National Cancer Institute Treatment Referral Center 9103. J Clin Oncol 11: 2405–2410, 1993
Stuart G, Bertelsen K, Mangioni C, Trope C, James K, Cassidy J, Kaye S, Timmers P, Roy JA, Piccart MJ: Updated analysis shows a highly significant improved overall survival for cisplatin-paclitaxel as first line treatment of advanced ovarian cancer: mature results of the EORTCGCCG, Nocova, NCIC CTG and Scottish intergroup trial. Proc Am Soc Clin Oncol 17: 361a, 1998 (Abstract)
McGuire WP, Hoskins WJ, Brady MF, Kucera PR, Partridge EE, Look KY, Clarke-Pearson DL, Davidson M: Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. N Engl J Med 334: 1–6, 1996
Lipton RB, Apfel SC, Dutcher JP, Rosenberg R, Kaplan J, Berger A, Einzig AI, Wiernik P, Schaumberg HH: Taxol produces a predominantly sensory neuropathy. Neurology 39: 368–373, 1989
Cersosimo RJ: Cisplatin neurotoxicity. Cancer Treat Rev 16: 195–211, 1989
Rowinsky EK, Chaudhry V, Forastiere AA, Sartorius SE, Ettinger DS, Grochow LB, Lubejko BG, Cornblath DR, Donehower RC: Phase I and pharmacologic study of paclitaxel and cisplatin with granulocyte colony-stimulating factor: neuromuscular toxicity is dose-limiting. J Clin Oncol 11: 2010–2020, 1993
Connelly E, Markman M, Kennedy A, Webster K, Kulp B, Peterson G, Belinson J: Paclitaxel delivered as a 3-hr infusion with cisplatin in patients with gynecologic cancers: unexpected incidence of neurotoxicity. Gynecol Oncol 62: 166–168, 1996
Piccart MJ, Bertelsen K, Stuart G, James K, Cassidy J, Kaye S, Hoctin Boes G, Timmers P, Roy JA, Pecorelli S: Is cisplatin-paclitaxel (P-T) the standard in first-line treatment of advanced ovarian cancer (Ov Ca)? The EORTC-GCCG, NOCOVA, NCI-C and Scottish intergroup experience. Proc Am Soc Clin Oncol 16: 352a, 1997 (Abstract)
Postma TJ, Vermorken JB, Liefting AJM, Pinedo HM, Heimans JJ: Paclitaxel-induced neuropathy. Ann Oncol 6: 489–494, 1995
Coukell AJ, Faulds D: Epirubicin. An updated review of its pharmacodynamic and pharmacokinetic properties and therapeutic efficacy in the management of breast cancer. Drugs 53: 453–482, 1997
Mollman JE, Glover DJ, Hogan WM, Furman RE: Cisplatin neuropathy. Risk factors, prognosis, and protection by WR-2721. Cancer 61: 2192–2195, 1988
Kemp G, Rose P, Lurain J, Berman M, Manetta A, Roullet B, Homesley H, Belpomme D, Glick J: Amifostine pretreatment for protection against cyclophosphamide-induced and cisplatin-induced toxicities: results of a randomized control trial in patients with advanced ovarian cancer. J Clin Oncol 14: 2101–2112, 1996
Chaudhry V, Rowinsky EK, Sartorius SE, Donehower RC, Comblath DR: Peripheral neuropathy from taxol and cisplatin combination chemotherapy: clinical and electrophysiological studies. Ann Neurol 35: 304–311, 1994
Wasserheit C, Frazein A, Oratz R, Sorich J, Downey A, Hochster H, Chachoua A, Wernz J, Zeleniuch-Jacquotte A, Blum R, Speyer J: Phase II trial of paclitaxel and cisplatin in women with advanced breast cancer: an active regimen with limiting neurotoxicity. J Clin Oncol 14: 1993–1999, 1996
Rowinsky EK, Gilbert MR, McGuire WP, Noe DA, Grochow LB, Forastiere AA, Ettinger DS, Lubejko BG, Clark B, Sartorius SE, Cornblath DR, Hendricks CB, Donehower RC: Sequences of taxol and cisplatin: a phase I and pharmacologic study. J Clin Oncol 9: 1692–1703, 1991
Cavaletti G, Bogliun G, Crespi V, Marzorati L, Zincone A, Marzola M, Rota S, Galli A, Tredici P, Tredici G: Neurotoxicity and ototoxicity of cisplatin plus paclitaxel in comparison to cisplatin plus cyclophosphamide in patients with epithelial ovarian cancer. J Clin Oncol 15: 199–206, 1997
Berger T, Malayeri R, Doppelbauer A, Krajnik G, Huber H, Auff E, Pirker R: Neurological monitoring of neurotoxicity induced by paclitaxel/cisplatin chemotherapy. Eur J Cancer 33: 1393–1399, 1997
Pirker R, Krajnik G, Z¨ochbauer S, Malayeri R, Kneussl M, Huber H: Paclitaxel/cisplatin in advanced non-small-cell lung cancer (NSCLC). Ann Oncol 6: 833–835, 1995
Neijt JP, Hansen M, Hansen SW, Sørensen PG, Sessa C, Witteveen PO, Engelholm SA, Stigaard L, Roer O, Lund B: Randomized phase III study in previously untreated epithelial ovarian cancer FIGO stage IIB, IIC, III, IV, comparing paclitaxel-cisplatin and paclitaxel-carboplatin. Proc Am Soc Clin Oncol 16: 352a, 1997 (Abstract)
Lissoni A, Gabriele A, Gorga G, Tumolo S, Landoni F, Mangioni C, Sessa C: Cisplatin-, epirubicin-, and paclitaxelcontaining chemotherapy in uterine adenocarcinoma. Ann Oncol 8: 969–972, 1997
Postma TJ, Heimans JJ, Muller MJ, Ossenkoppele GJ, Vermorken JB, Aaronson NK: Pitfalls in grading severity of chemotherapy-induced peripheral neuropathy. Ann Oncol 9: 739–744, 1998
Siegal T, Haim N: Cisplatin-induced peripheral neuropathy. Frequent off-therapy deterioration, demyelinating syndromes, and muscle cramps. Cancer 66: 1117–1123, 1990
Rowinsky EK, Donehower RC: Paclitaxel (Taxol). N Engl J Med 332: 1004–1014, 1995
Van Gerven JMA, Moll JWB, van den Bent MJ, Bontenbal M, van der Burg MEL, Verweij J, Vecht ChJ: Paclitaxel (Taxol) induces cumulative mild neurotoxicity. Eur J Cancer 30A: 1074–1077, 1994
Gregg RW, Molepo JM, Monpetit VJA, Mikael NZ, Redmond D, Gadia M, Stewart DJ: Cisplatin neurotoxicity: the relationship between dosage, time, and platinum concentration in neurologic tissues, and morphologic evidence of toxicity. J Clin Oncol 10: 795–803, 1992
R¨oytt¨a M, Raine CS: Taxol-induced neuropathy: chronic effects of local injection. J Neurocytol 15: 483–496, 1986
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Postma, T., Hoekman, K., van Riel, J. et al. Peripheral Neuropathy Due to Biweekly Paclitaxel, Epirubicin and Cisplatin in Patients with Advanced Ovarian Cancer. J Neurooncol 45, 241–246 (1999). https://doi.org/10.1023/A:1006343818656
Issue Date:
DOI: https://doi.org/10.1023/A:1006343818656