Abstract
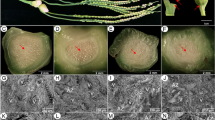
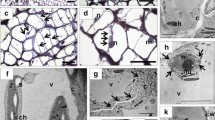
Fruitlet abscission during fruit development is due to the activation ofpre-differentiated abscission zones (AZs) located between twig andpedicel, and/or pedicel and pericarp. Major advances on biochemicaland molecular aspects are related to β-1,4-endoglucanase (EG) andpolygalacturonase (PG), two cell hydrolases involved in the cell walldisassemblement responsible for fruit shedding. AZ activation isaccompanied by an increase in activity and transcript accumulation ofone or both enzymes. Expression of PG genes specifically related toabscission has been found in tomato flower AZ. In peach, an EG genehighly expressed in leaf and fruitlet AZs has been isolated. AZactivation is preceded by an induction of ethylene biosynthesis,paralleled by a stimulation of ACO activity and transcript accumulation.Ethylene, besides a dramatic stimulation of PG and EG, up or downregulates several other abscission related genes. The specificexpression of genes encoding for ethylene receptors in the AZ wouldsupport the hypothesis that fruitlet AZ specificity may depend on theability of this region to sense ethylene.
Similar content being viewed by others
References
Bargioni G and Ramina A (1972) Aspetti anatomici ed istochimici del distacco dei frutti nella cv di ciliegio dolce “Vittoria”. Rivista Ortiflorofrutticoltura Italiana 1: 20-26
Blume B and Grierson D (1997) Expression of ACC oxidase promoter-GUS fusions in tomato and Nicotiana plumbaginifolia regulated by developmental and environmental stimuli. Plant J 12: 731-746
Bonghi C, Rascio N, Ramina A and Casadoro G (1992) Cellulase and polygalacturonase involvement in the abscission of leaf and fruit explants of peach. Plant Mol Biol 20: 839-848
Bonghi C, Ferrarese L, Ruperti B, Tonutti P and Ramina A (1998) Endo-β-1,4 glucanases are involved in peach fruit growth and ripening, and regulated by ethylene. Physiol Plant 102: 346-352
Brummel DA, Hall BD and Bennett AB (1999) Antisense suppression of tomato endo-1,4 β-glucanase Cel2 mRNA accumulation and increase the force required to break fruit abscission zone but does not affect fruit softening. PlantMolec Biol 40: 615-622
Cass LG, Kirven KA and Christoffersen RE (1990) Isolation and characterization of cellulase gene family member expressed during avocado fruit ripening. Mol Gen Genet 223: 76-86
Chang C, Shing F, Bleecker A and Meyerowitz E (1993) Arabidopsis ethylene-response gene ETR1: Similarity of product to two component regulators. Science 262: 539-544
Davenport TL and Manners M (1983) Nucellar senescence and ethylene production as they relate to avocado fruitlet abscission. J Exp Bot 33: 815-825
del Campillo E, Durbin M and Lewis LN (1988) Changes in two forms of membrane-associated cellulase during ethyleneinduced abscission. Plant Physiol 88: 904-909
del Campillo E and Bennett AB (1996). Pedicel breakstrenght and cellulase gene expression during tomato flower abscission. Plant Physiol 111: 813-820
Ecker JR (1995) The ethylene signal transduction pathway in plants. Science 268: 667-675
Ferrarese L, Trainotti L, Moretto P, Polverino de Laureto P, Rascio N and Casadoro G (1995) Differential ethyleneinducible expression of cellulase in pepper plant. Plant Mol Biol 29: 278-289
Greenberg J, Goren R and Riov J (1975). The role of cellulase and polygalacturonase in abscission of young and mature Shamouti orange fruits. Physiol Plant 34: 1-7
Kalaitzis P, Koehler SM and Tucker ML (1995) Cloning of a tomato polygalacturonase expressed in abscission. Plant Mol Biol 28: 647-656
Kalaitzis P, Solomos T and Tucker ML (1997) Three different polygalacturonases are expressed in tomato leaf and flower abscission, each with a different temporal expression pattern. Plant Physiol 113: 1303-1308
Kanellis AK and Kalaitzis P (1992) Cellulase occurs in multiple active forms in ripe avocado fruit mesocarp. Plant Physiol 98: 530-534
Kieber J (1997) The ethylene signal transduction pathway in Arabidopsis. J Exp Bot 48: 211-218
Lashbrook CC, Gonzalez-Bosch C and Bennett AB (1994) Two divergent endo-β-1,4-glucanase genes exhibit overlapping expression in ripening fruit and abscissing flowers. Plant Cell 6: 1485-1493
Lashbrook CC, Giovannoni JJ, Hall BD, Fischer RL and Bennett AB (1998) Transgenic analysis of tomato endo-1,4 42_-glucanase gene function. Role of cel1 in floral abscission. Plant J 13: 303-310
Lashbrook CC, Tieman D and Klee HJ (1998) Differential regulation of the tomato ETR gene family throughout plant development. Plant J 15: 243-252
Lee E, Speirs J, Gray J and Brady CJ (1990) Homologies to the tomato endopolygalacturonase gene in peach genome. Plant Cell Environ 13: 516-521
McManus MT and Osborne DJ (1989) Identification of leaf abscission zone as a specific class of target cells for ethylene. In: Osborne DJ and Jackson MB (eds) Cell Separation in Plants. NATO ASI Series, Vol 35. Heidelberg: Springer-Verlag Berlin, pp 201-210
Miller AN, Krizek BA and Walsh CS (1988) Whole fruit ethylene evolution and ACC content. J Amer Soc Hort Sci 113: 119-124
Nunez-Elisea R and Davenport TL (1986) Abscission of mango fruitlets as influenced by enhanced ethylene biosynthesis. Plant Physiol 82: 991-994
Pandita VK and Jindal KK (1991). Enzymatic and anatomical changes in abscission zone cells of apple fruits induced by ethephon. Biol Plant 33: 20-25
Payton S, Fray RG, Brown S and Grierson D (1996) Ethylene receptor expression is regulated during fruit ripening, flower senescence and abscission. Plant Mol Biol 31: 1227-1231
Ramina A, Bonghi C, Giovannoni JJ, Ruperti B and Tonutti P (1999) Differential display and isolation of cDNAs corresponding to mRNAs whose abundance is influenced by ethylene during peach fruitlet abscission. In: Kanellis AK, Chang C, Klee H, Bleecker AB, Pech JC and Grierson D (eds). Biology and biotechnology of the plant hormone ethylene II. pp 249-254. Kluwer Academic Publishers. Dordrecht, Boston, London
Ramina A, Rascio N and Masia A (1989) The abscission process in peach: structural, biochemical and hormonal aspects. In: Osborne DJ and Jackson MB (eds) Cell Separation in Plants. Berlin: Springer Verlag, pp 233-240
Rascio N, Casadoro G, Ramina A and Masia A (1985) Structural and biochemical aspect of peach fruit abscission (Prunus persica L. Batsch). Planta 164: 1-11
Reid MS (1985) Ethylene and abscission. HortScience 20: 45-50
Ruperti B, Bonghi C, Tonutti P and Ramina A (1998) Ethylene biosynthesis in peach fruitlet abscission. Plant Cell Environ 21: 731-737
Schaller GE and Bleecker AB (1995) Ethylene binding sites generated in yeast expressing in Arabidopsis Etrl gene. Science 270: 1809-1811
Sexton R, Palmer JM, Whyte NA and Littlejhons S (1997) Cellulase, fruit softening and abscission in red raspberry Rubus idaeus L. cv Glen Clova. Ann Bot 80: 371-376
Stösser R, Rasmussen HP and Bukovac MJ (1969) Hystochemical changes in the developing abscission layer in fruit of Prunus cerasus L. Planta 86: 151-164
Taylor JE, Tucker GA, Lasslett Y, Smith CJS, Arnold CM, Watson CF, Schuch W, Grierson D and Roberts JA (1990) Polygalacturonase expression during leaf abscission of normal and transgenic tomato plants. Planta 183: 133-138
Tonutti P, Cass L and Christoffersen RE (1995) The expression of cellulase gene family members during induced avocado fruit abscission and ripening. Plant Cell Environ 18: 709-713
Tonutti P, Bonghi C, Ruperti B and Ramina A (1997) The modulation of ethylene biosynthesis and ACC oxidase gene expression during peach fruit development and fruitlet abscission. In: Kanellis AK, Chang C, Kende H, Grierson D (eds) Biology and Biotechnology of the Plant Hormone Ethylene. NATO ASI Series, Vol 3/34. Dordrecht/Boston/London: Kluwer Academic Publisher, pp 149-153
Tonutti P, Bonghi C, Ruperti B, Scapin A and Ramina A (1999) An Arabidopsis ETR1 homologue is constitutively expressed in peach fruit abscission zone and mesocarp. In: Kanellis AK, Chang C, Klee H, Bleecker AB, Pech JC and Grierson D (eds). Biology and biotechnology of the plant hormone ethylene II. pp. 267-268. Kluwer Academic Publishers. Dordrecht, Boston, London
Trainotti L, Rascio N and Casadoro G (1993) Expression of an endopolygalacturonase gene during growth and abscission of peach. Hereditas 119: 301-304
Trainotti L, Spolaore S, Farrarese L and Casadoro G (1997) Characterization of ppEG1, a member of a multigene family which encodes endo-β-1,4-glucanase in peach. Plant Mol Biol 34: 791-802
Zhong GY, Riov Y, Goren R and Holland D (1996) Isolation of novel genes from citrus abscission zone that are highly homologous to genes encoding for eukaryotic protein kinases. Abstract of Workshop on Biology and Biotechnology of the Plant Hormone Ethylene. Chania, Crete Greece. pp 89
Zhou D, Kalaitzis P, Mattoo AK and Tucker ML (1996) The mRNA for an ETR1 homologue in tomato is constitutively expressed in vegetative and reproductive tissues. Plant Mol Biol 30: 1331-1338
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bonghi, C., Tonutti, P. & Ramina, A. Biochemical and molecular aspects of fruitlet abscission. Plant Growth Regulation 31, 35–42 (2000). https://doi.org/10.1023/A:1006338210977
Issue Date:
DOI: https://doi.org/10.1023/A:1006338210977