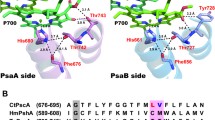
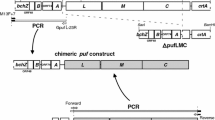
Abstract
Primary charge separation within Photosystem II (PS II) is much slower (time constant ∼ 21 ps) than the equivalent step in the related reaction center (RC) found in purple bacteria (∼ 3 ps). In the case of the bacterial RC, replacement of a specific tyrosine residue within the M subunit (at position 210 in Rhodobacter sphaeroides), by a leucine residue slows down charge separation to ∼ 20 ps. Significantly the analogous residue in PS II, within the D2 polypeptide, is a leucine not a tyrosine (at position D2-205, Chlamydomonas reinhardtii numbering). Consequently, it has been postulated [Hastings et al. (1992) Biochemistry 31: 7638–7647] that the rate of electron transfer could be increased in PS II by replacing this leucine residue with tyrosine. We have tested this hypothesis by constructing the D2-Leu205Tyr mutant in the green alga, Chlamydomonas reinhardtii, through transformation of the chloroplast genome. Primary charge separation was examined in isolated PS II RCs by time-resolved optical spectroscopy and was found to occur with a time constant of 40 ps. We conclude that mutation of D2-Leu205 to Tyr does not increase the rate of charge separation in PS II. The slower kinetics of primary charge separation in wild type PS II are probably not due to a specific difference in primary structure compared with the bacterial RC but rather a consequence of the P680 singlet excited state being a shallower trap for excitation energy within the reaction center.
Similar content being viewed by others
References
Alizadeh S, Nixon PJ, Telfer A and Barber J (1995) Isolation and characterisation of the Photosystem II reaction center complex from a double mutant of Chlamydomonas reinhardtii. Photosynth Res 43: 165–171
Allen JP, Feher G, Yeates TO, Komiya H and Rees DC (1987) Structure of the reaction center from Rhodobacter sphaeroides R-26. Proc Natl Acad USA 84: 6162–6166
Andronis C (1996) Site-directed mutagenesis of the D2 protein in the green alga Chlamydomonas reinhardtii. PhD Thesis, University of London
Andronis C, Kruse O, Deák Z, Vass I, Diner BA and Nixon PJ (1998) Mutation of residue threonine-2 of the D2 polypeptide and its effect on Photosystem II function in Chlamydomonas reinhardtii. Plant Physiol 117: 515–524
Arnon DI (1949) Copper enzymes in isolated chloroplasts. Polyphenol oxidase in Beta vulgaris. Plant Physiol 24: 1–15
Beekman LMP, Van Stokkum IHM, Monshouwer R, Rijnders AJ, McGlynn P, Visschers RW, Jones MR and Van Grondelle R (1996) Primary electron transfer in membrane-bound reaction centers with mutations at the M210 position. J Phys Chem 100: 7256–7268
Boynton JE and Gillham NW (1993) Chloroplast transformation in Chlamydomonas. Methods Enzymol 217: 510–536
Chan CK, Chen LXQ, DiMagno TJ, Hanson DK, Nance SL, Schiffer M, Norris JR and Fleming GR (1991) Initial electron transfer in photosynthetic reaction centers of Rhodobacter capsulatus mutants. Chem Phys Lett 176: 366–372
Choquet Y, Goldschmidt-Clermont M, Girard-Bascou J, Kück U, Bennoun P and Rochaix J-D (1988) Mutant phenotypes support a trans-splicing mechanism for the expression of the tripartite psaA gene in the C. reinhardtii chloroplast. Cell 52: 903–913
Chua NH, Matlin K and Bennoun P (1975) A chlorophyll-protein complex lacking in Photosystem I mutants of Chlamydomonas reinhardtii. J Cell Biol 67: 361–377
Danon A and Mayfield SP (1994) Light-regulated translation of chloroplast messenger RNAs through redox potential. Science 266: 1717–1719
Deisenhofer J, Epp O, Miki K, Huber R and Michel H (1985) Structure of the protein subunits in the photosynthetic reaction centre of Rhodospeudomonas viridis at 3 Å resolution. Nature 318: 618–624
Deisenhofer J, Epp O, Sinning I and Michel H (1995) Crystallographic refinement at 2.3 Å resolution and refined model of the photosynthetic reaction centre form Rhodopseudomonas viridis. J Mol Biol 246: 429–457
Donovan B, Walker LA, Yocum CF and Sension RJ (1996) Transient Absorption studies of primary charge separation in Photosystem II. J Phys Chem B 100: 1945–1949
Durrant JR, Hastings G, Joseph DM, Barber J, Porter G and Klug DR (1993) Rate of oxidation of P680 in isolated Photosystem II reaction centres monitored by loss of chlorophyll stimulated emission. Biochemistry 32: 8259–8267
Durrant JR, Hastings G, Joseph DM, Barber J, Porter G and Klug DR (1992) Subpicosecond equilibration of excitation energy in isolated Photosystem II reaction centers. Proc Natl Acad USA 89: 11632–11636
Durrant JR, Klug DR, Kwa SLS, Van Grondelle R, Porter G and Dekker J P (1995) Proc Natl Acad USA 92: 4798–4802
Durrant JR, Nixon PJ, Barber J and Klug DR (1999) In: Current Research in Photosynthesis, Kluwer Academic Publishers, Dordrecht, The Netherlands (in press)
Finkele U, Lauterwasser C, Zinth W, Gray KA and Oesterhelt D (1990) Role of tyrosine M210 in the initial charge separation of reaction centres of Rhodobacter spaeroides. Biochemistry 29: 8517–8521
Giorgi LB, Durrant JR, Alizadeh S, Nixon PJ, Joseph DM, Rech T, Barber J, Porter G and Klug DR (1994) Comparison of primary electron transfer in Photosystem II reaction centres isolated from the higher plant Pisum sativum and the green alga Chlamydomonas reinhardtii. Biochim Biophys Acta 1186: 247–251
Giorgi LB, Nixon PJ, Merry SAP, Joseph DM, Durrant JR, De Las Rivas J, Barber J, Porter G and Klug DR (1996) Comparison of primary charge separation in the Photosystem II reaction centre complex isolated from wild-type and D1–130 mutants of the cyanobacterium Synechocystis PCC 6803. J Biol Chem 271: 2093–2101
Goldschmidt-Clermont M (1991) Transgenic expression of aminoglycoside adenine transferase in the chloroplast: A selectable marker for site-directed transformation of Chlamydomonas. Nucleic Acids Res 19: 4083–4089
Gray KA, Farchaus JW, Wachtveitl J, Breton J and Oesterhelt D (1990) Initial characterisation of site-directed mutants of tyrosine M210 in the reaction centre of Rhodobacter sphaeroides. EMBO J 9: 2061–2070
Greenfield SR, Seibert M, Govindjee and Wasielewski MR (1997) Direct measurement of effective rate constant for primary charge separation in isolated Photosystem II reaction centres. J Phys Chem 101: 2251–2255
Hankamer B, Barber J and Boekema E (1997) Structure and membrane organization of Photosystem II in green plants. Annu Rev Plant Physiol Plant Mol Biol 48: 641–671
Harris EH (1989) The Chlamydomonas Sourcebook. Academic Press, New York
Hastings G, Durrant JR, Hong Q, Barber J, Porter G and Klug DR (1992) Observation of pheophytin reduction in Photosystem II reaction centres using femtosecond absorption spectroscopy. Biochemistry 31: 7638–7647
Holzapfel W, Finkele U, Kaiser W, Oesterhelt D, Scheer H, Stilz HU and Zinth W (1990) Electron transfer in the reaction center from Rhodobacter sphaeroides. Proc Natl Acad USA 87: 5168–5172
Jones MR, Heer-Dawson M, Mattioli TA, Hunter CN and Robert B (1994) Site-specific mutagenesis of the reaction centre from Rhodobacter sphaeroides studied by Fourier transform Raman spectroscopy: mutations at tyrosine M210 do not affect the electronic structure of the primary donor. FEBS Lett 339: 18–24
Kirmaier C and Holten D (1993) In: Deisenhofer J and Norris JR (eds) The Photosynthetic Reaction Center, Vol 2, pp 49–70. Academic Press, San Diego, California
Klug DR, Durrant JR and Barber J (1998) The entanglement of excitation energy transfer and electron transfer in the reaction centre of Photosystem II. Phil Trans R Soc London A 356: 449–464
Klug DR, Rech T, Joseph DM, Barber J, Durrant JR and Porter G (1995) Primary processes in isolated Photosystem II reaction centres probed by magic angle transient absorption spectroscopy. Chem Phys 194: 433–442
Kück U, Choquet Y, Schneider M, Dron M and Bennoun P (1987) Structural and transcription analysis of two homologous genes for the P700 chlorophyll a-apoproteins in Chlamydomonas reinhardtii: evidence for in vivo trans-splicing. EMBO J 6: 2185–2195
Kumazaki S, Joseph DM, Crystall B, Tachibana Y, Durrant J, Barber J, Porter G, Yoshihara K and Klug DR (1996) In: Mathis P (ed) Photosynthesis: From Light to Biosphere, pp 883–886. Kluwer Academic Publishers, Dordrecht, The Netherlands
Kunkel TA, Roberts JD and Zakour RA (1987) Rapid and efficient site-directed mutagenesis without phenotypic selection. Methods Enzymol 154: 367–382
Merry SAP, Nixon PJ, Barter LMC, Schilstra M, Porter G, Barber J, Durrant JR and Klug DR (1998) Modulation of quantum yield of primary radical pair formation in Photosystem II by site-directed mutagenesis affecting radical cations and anions. Biochemistry 37: 17439–17447
Michel H and Deisenhofer J (1988) Relevance of the photosynthetic reaction center from purple bacteria to the structure of Photosystem II Biochemistry 27: 1–7
Müller MG, Hucke M, Reus M and Holzwarth AR (1996) Primary processes and structure of the Photosystem II reaction center. 4. Low intensity femtosecond transient absorption spectra of D1-D2-cyt-b559 reaction center. J Phys Chem 100: 9527–9536
Nagarajan V, Parson WW, Gaul D and Schenk C (1990) Effect of specific mutations of tyrosine-(M)210 on the primary photosynthetic electron-transfer process in Rhodobacter sphaeroides. Proc Natl Acad Sci USA 87: 7888–7892
Nanba O and Satoh K (1987) Isolation of a Photosystem II reaction center containing D1 and D2 polypeptide and cytochrome b559. Proc Natl Acad Sci USA 84: 109–112
Nickelsen J, Van Dillewijn J, Rahire M and Rochaix J-D (1994) Determinants for stability of the chloroplast psbD RNA are located within its short leader region in Chlamydomonas reinhardtii. EMBO J 13: 3182–3191
Nixon PJ, Chisholm DA and Diner BA (1992) In: Shewry PR and Gutteridge S (eds) Plant Protein Engineering, pp 93–141. Cambridge University Press, Cambridge, UK
Nugent JHA (1996) Oxygenic photosynthesis. Electron transfer in Photosystem I and Photosystem II. Eur J Biochem 237: 519–531
Pakrasi HB (1995) Genetic analysis of the form and function of Photosystem I and Photosystem II. Ann Rev Genet 29: 755–776
Parson WW, Chu Z-T and Warshel A (1990) Electrostatic control of charge separation in bacterial photosynthesis. Biochim Biophys Acta 1012: 251–272
Rech T, Durrant JR, Joseph DM, Barber J, Porter G and Klug DR (1994) Does slow energy transfer limit the observed time constant for radical pair formation in Photosystem II reaction centres? Biochemistry 33: 14768–14774.
Rhee K-H, Morris EP, Barber J and Kühlbrandt W (1998) Threedimensional structure of the plant Photosystem II reaction centre at 8ÅA resolution. Nature 396: 283–286
Rhee K-H, Morris EP, Zheleva D, Hankamer B, Kühlbrandt W and Barber J (1997) Two-dimensional structure of plant Photosystem II at 8 ÅA resolution. Nature 389: 522–526
Ruffle SV, Donnelly D, Blundell TL and Nugent JHA (1992) A three-dimensional model of the Photosystem II reaction centre of Pisum sativum. Photosynth Res 34: 287–300
Rutherford AW (1986) How close is the analogy between the reaction center of Photosystem II and that of purple bacteria. Biochem Soc Trans 14: 15–17
Sambrook J, Fritsch EF and Maniatis J (1989) Molecular Cloning: A Laboratory Manual (2nd ed). Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York
Schiffer M and Norris JR (1993) In: Deisenhofer J and Norris JR (eds) The Photosynthetic Reaction Center, Vol 1, pp 1–12. Academic Press, San Diego, California
Shochat S Arlt T, Francke C, Gast P, Van Noort PI, Otte SCM, Schelvis HPM, Schmidt S, Vijgenboom E, Vrieze J, Zinth W and Hoff AJ (1994) Spectroscopic characterization of reaction centers of the (M)210 mutant of the photosynthetic bacterium Rhodobacter sphaeroides. Photosynth Res 40: 55–66
Svensson B, Etchebest C, Tuffery P, Van Kan P, Smith J and Styring S (1996) A model for the Photosystem II reaction center core including the structure of the primary donor P680. Biochemistry 35: 14486–14502
Van Brederode ME, Jones MR, Van Mourik F, Van Stokkum IHM and Van Grondelle R (1997) A new pathway for transmembrane electron transfer in photosynthetic reaction centres of Rhodobacter sphaeroides not involving the excited special pair. Biochemistry 36: 6855–6861
Zheleva D, Hankamer B and Barber J (1996) Heterogeneity and pigment composition of isolated Pphotosystem II reaction centers. Biochemistry 35: 15074–15079
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Andronis, C., Merry, S.A., Durrant, J.R. et al. Mutation of the Chlamydomonas reinhardtii analogue of residue M210 of the Rhodobacter sphaeroides reaction center slows primary electron transfer in Photosystem II. Photosynthesis Research 62, 205–217 (1999). https://doi.org/10.1023/A:1006331714914
Issue Date:
DOI: https://doi.org/10.1023/A:1006331714914