Abstract
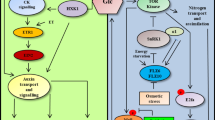
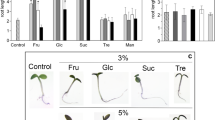
Glucose effects on the expression of the abscisic acid-inducible Rab16A gene were examined in rice and barley embryos. Glucose feeding to rice embryos negatively affects the endogenous abscisic acid content and represses the promoter activity of the Rab16A gene. Glucose repression of the Rab16A gene takes place both at a transcriptional and a post-transcriptional level. Modulation of the abscisic acid content in rice embryos triggered by glucose did not directly influence the expression of the rice α-amylase gene RAmy3D, which is known to be under glucose control. The possible interaction between the glucose and abscisic acid signaling pathway is discussed.
Similar content being viewed by others
References
Baker, S., Harada, J. and Goldberg, R. 1988. Cellular localization of soybean storage protein mRNA in transformed tobacco seed. Proc. Natl. Acad. Sci. USA 85: 458–462.
Bray, E.A. 1993. Molecular responses to water deficit. Plant Physiol. 103: 1035–1040.
Busk, P.K. and Pagè s, M. 1998. Regulation of abscisic acid-induced transcription. Plant Mol. Biol. 37: 425–435.
Bustos, M.M., Iyer, M. and Gagliardi, S.J. 1998. Induction of a β-phaseolin promoter by exogenous abscisic acid in tobacco: developmental regulation and modulation by external sucrose and Ca2+ ions. Plant Mol. Biol. 37: 265–274.
Chen, M.-T. and Yu, S.-M. 1998. The 30 untranslated region of rice α-amylase gene functions as a sugar-dependent mRNA stability determinant. Proc. Natl. Acad. Sci. USA 95: 6543–6547.
DeWald, D.B., Sadka, A. and Mullet, J.E. 1994. Sucrose modulation of soybean Vsp gene expression is inhibited by auxin. Plant Physiol. 104: 439–444.
Dure, L., III, Crouch, M., Harada, J., Ho, T.-H.D., Mundy, J., Quatrano, R., Thomas, T. and Sung, Z.R. 1989. Common amino acid sequence domains among the LEA proteins of higher plants. Plant Mol. Biol. 12: 475–486.
Guglielminetti, L., Perata, P. and Alpi, A. 1995. Effect of anoxia on carbohydrate metabolism in rice seedlings. Plant Physiol. 108: 735–741.
Guiltinan, M.J., Marcotte, J., William, R. and Quatrano, R.S. 1990. A plant leucine zipper protein that recognizes an abscisic acid response element. Science 250: 267–270.
Hattori, T. and Hobo, T. 1999. Regulation of gene expression by abscisic acid in rice. In: K. Shimamoto (Ed.), Molecular Biology of Rice, Springer-Verlag, Tokyo, pp. 139–160.
Huang, N., Koizumi, N., Reinl, S. and Rodriguez, R.L. 1990. Structural organization and differential expression of rice α-amylase genes. Nucl. Acids Res. 18: 7007–7014.
Hwang, Y.-S., Karrer, E.E., Thomas, B.R., Chen, L. and Rodriguez, R.L. 1998. Three cis-elements required for rice α-amylase Amy3D expression during sugar starvation. Plant. Mol. Biol. 36: 331–341.
Ingram, J. and Bartels, D. 1996. Molecular basis of dehydration tolerance in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 47: 377–403.
Izawa, T., Foster, R. and Chua, N.-H. 1993. Plant bZIP protein DNA binding specificity. J. Mol. Biol. 230: 1131–1144.
Jang, J.C., Leon, P., Zhou, L. and Sheen, J. 1997. Hexokinase as a sugar sensor in higher plants. Plant Cell 9: 5–19.
Jensen, A.B., Goday, A., Figueras, M., Jessop, A.C. and Pagè s, M. 1998. Phosphorylation mediates the nuclear targeting of the maize Rab17 protein. Plant J. 13: 691–697.
Koch, K.E. 1996. Carbohydrate-modulated gene expression in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 47: 509–540.
Lanahan, M.B., Ho, T.-H.D., Rogers, S.W. and Rogers, J.C. 1992. A gibberellin response complex in cereal α-amylase gene promoters. Plant Cell 4: 203–211.
Lu, C.-A., Lim, E.-K. and Yu, S.-M. 1998. Sugar response sequence in the promoter of a rice α-amylase gene serves as a transcriptional enhancer. J. Biol. Chem. 273: 10120–10131.
Mitsuhara, I., Ugaki, M., Hirochika, H., Ohshima, M., Murakami, T., Gotoh, Y., Katayose, Y., Nakamura, S., Honkura, R., Nishimiya, S., Ueno, K., Mochizuki, A., Tanimoto, Y., Tsugawa, H., Otsuki, Y. and Ohashi, Y. 1996. Efficient promoter cassettes for enhanced expression of foreign genes in dicotyledonous and monocotyledonous plants. Plant Cell Physiol. 37: 49-59.
Mitsunaga, S., Rodriguez, R.L. and Yamaguchi, J. 1994. Sequencespecific interactions of a nuclear protein factor with the promoter region of a rice gene for α-amylase, RAmy3D. Nucl. Acids Res. 22: 1948–1953.
Morita, A., Umemura, T., Kuroyanagi, M., Futsuhara, Y., Perata, P. and Yamaguchi, J. 1998. Functional dissection of a sugarrepressed α-amylase gene (RAmy1A) promoter in rice embryos. FEBS Lett. 423: 81–85.
Mundy, J. and Chua, N.-H. 1988. Abscisic acid and water-stress induce the expression of a novel rice gene. EMBO J. 7: 2279–2286.
Murashige, T. and Skoog, F. 1962. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 15: 473–497.
Nakagawa, H., Ohmiya, K. and Hattori, T. 1996. A rice bZIP protein, designated OSBZ8, is rapidly induced by abscisic acid. Plant J. 9: 217–227.
Neill, S.J., Horgan, R. and Rees, A.F. 1987. Seed development and vivipary in Zea mays L. Planta 171: 358–364.
Oeda, K., Salinas, J. and Chua, N.-H. 1991. A tobacco bZip transcription activator (TAF-1) binds to a G-box-like motif conserved in plant genes. EMBO J. 10: 1793–1802.
Ohto, M., Nakamura-Kito, K. and Nakamura, K. 1992. Induction of expression of genes coding for sporamin and β-amylase by polygalacturonic acid in leaf-petiole cuttings of sweet potato. Plant Physiol. 99: 422–427.
Ono, A., Izawa, T., Chua, N.-H. and Shimamoto, K. 1996. The rab16B promoter of rice contains two distinct abscisic acidresponsive elements. Plant Physiol. 112: 483–491.
Perata, P., Matsukura, C., Vernieri, P. and Yamaguchi, J. 1997. Sugar repression of gibberellin-dependent signaling pathway in barley embryos. Plant Cell 9: 2197–2208.
Radley, M. 1967. Site of production of gibberellin-like substances in germinating barley embryos. Planta 75: 164–171.
Rook, F., Gerrits, N., Kortstee, A, van Kampen, M., Borrias, M., Weisbeek, P. and Smeekens, S. 1998. Sucrose-specific signalling represses translation of the Arabidopsis ATB2 bZIP transcription factor gene. Plant J. 15: 253–263.
Shen, Q. and Ho, T.-H.D. 1995. Functional dissection of an abscisic acid (ABA)-inducible gene reveals two independent ABAresponsive complexes each containing a G-Box and a novel cis-acting element. Plant Cell 7: 295–307.
Shen, Q, Zhang, P. and Ho, T.-H.D. 1996. Modular nature of abscisic acid (ABA) response complexes: composite promoter units that are necessary and sufficient for ABA induction of gene expression in barley. Plant Cell 8: 1107–1119.
Skadsen, R.W. 1993. Aleurones from a barley with low α-amylase activity become highly responsive to gibberellic acid when detached from the starchy endosperm. Plant Physiol. 102: 195–203.
Skriver, K. and Mundy, J. 1990. Gene expression in response to abscisic acid and osmotic stress. Plant Cell 2: 503–512.
Skriver, K., Olsen, F.L., Rogers, J.C. and Mundy, J. 1991. Cis-acting DNA elements responsive to gibberellin and its antagonist abscisic acid. Proc. Natl. Acad. Sci. USA 88: 7266–7270.
Smeekens, S. 1998. Sugar regulation of gene expression in plants. Curr. Opin. Plant Biol. 1: 230–234.
Tanaka, A., Mita, S., Ohta, S., Kyozuka, J., Shimamoto, K. and Nakamura, K. 1990. Enhancement of foreign gene expression by a dicot intron in rice but not in tobacco is correlated with an increased level of mRNA and an efficient splicing of the intron. Nucl. Acids Res. 18: 6767-6770.
Toyofuku, K., Umemura, T. and Yamaguchi, J. 1998. Promoter elements required for sugar-repression of the RAmy3D gene for α-amylase in rice. FEBS Lett. 428: 275–280.
Umemura, T., Perata, P., Futsuhara, Y. and Yamaguchi, J. 1998. Sugar sensing and α-amylase gene repression in rice embryos. Planta 204: 420–428.
Vernieri, P., Perata, P., Armellini, D., Bugnoli, M., Presentini, R., Lorenzi, R., Ceccarelli, N., Alpi, A. and Tognoni, F. 1989. Solid phase radioimmunoassay for the quantitation of abscisic acid in 460 plant crude extracts using a new monoclonal antibody. J. Plant Physiol. 134: 441–446.
Walker-Simmons, M., Reaney, M. Quarrie, S., Perata, P., Vernieri, P. and Abrams, S. 1990. Monoclonal antibody recognition of abscisic acid analogs. Plant Physiol. 95: 46–51.
Williams, M.E., Foster, R. and Chua, N.-H. 1992. Sequences flanking the hexameric G-box core CACGTG affect the specificity of protein binding. Plant Cell 4: 485–496.
Wingler, A., von Schaewen, A., Leegood, R.C., Lea, P.J. and Quick, P. 1998. Regulation of leaf senescence by cytokinin, sugar, and light. Plant Physiol 116: 329–335.
Yamaguchi, J. 1998. Analysis of embryo-specific α-amylase using isolated mature rice embryos. Breed Sci. 48: 365–370.
Yamaguchi-Shinozaki, K., Mundy, J. and Chua, N.-H. 1989. Four tightly linked rab genes are differentially expressed in rice. Plant Mol. Biol. 14: 29–39.
Zeevaart, J.A.D. and Creelman, R.A. 1988. Metabolism and physiology of abscisic acid. Annu. Rev. Plant Physiol. PlantMol. Biol. 39: 439–473.
Zhou, L., Jang, J.-C., Jones, T.L. and Sheen, J. 1998. Glucose and ethylene signal transduction crosstalk revealed by an Arabidopsis glucose-insensitive mutant. Proc. Natl. Acad. Sci. USA 95: 10294–10299.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Toyofuku, K., Loreti, E., Vernieri, P. et al. Glucose modulates the abscisic acid-inducible Rab16A gene in cereal embryos. Plant Mol Biol 42, 451–460 (2000). https://doi.org/10.1023/A:1006318117107
Issue Date:
DOI: https://doi.org/10.1023/A:1006318117107