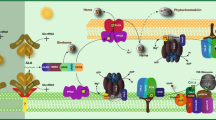
Abstract
A critical review of studies on import of Lhcb (apoproteins of LHC II) by chloroplasts uncovered a mechanism for initiation of assembly of light-harvesting complexes. Manipulation of in vivo systems and mutagenesis of specific residues in the protein showed that accumulation of physiological amounts of Lhcb by the plastid requires interaction of the protein with Chl within the inner membrane of the chloroplast envelope. ‘Retention motifs’, commonly -EXXHXR- in the first membrane-spanning region (helix-1) and -EXXNXR- in the third membrane-spanning region (helix-3), occur in the primary sequence of the protein. Mutations in these sequences prevent accumulation of Lhcb by isolated chloroplasts. We propose that the His or Asn sidechain and a transient intrahelix ion-pair with the Glu and Arg residues provide ligands for two molecules of Chl in each motif, which serve as a sensing mechanism for the availability of Chl. Interaction of two Chl molecules with both motifs is required for stable insertion of the protein into the membrane. Chl(ide) is possibly quenched by interaction with xanthophylls immediately after synthesis, and Chl-lutein pairs may initiate folding of Lhcb. Lhcb that does not immediately interact with sufficient Chl molecules is not retained by the organelle and, in vivo, is retracted into the cytosol or shunted to vacuoles for degradation rather than imported into the plastid stroma. The ubiquitous existence of retention motifs from small Lhcb-like polypeptides in cyanobacteria to all nuclear-encoded Chl-binding proteins (the Lhcb and Lhca families and related proteins) testify to the importance of these sequences in assembly of Chl-protein complexes.
Similar content being viewed by others
References
Adamska I (1997) ELIPS – light-induced stress proteins. Physiol Plant 100: 794–805
Adamska I, Kloppstech K and Ohad I (1993) Early light-inducible protein in pea is stable during light stress but is degraded during recovery at low light intensity. J Biol Chem 268: 5438–5444
Akoyunoglou G and Argyroudi-Akoyunoglou JH (1978) Control of thylakoid growth in Phaseolus vulgaris. Plant Physiol 61: 834–837
Allen KD and Staehelin LA (1994) Polypeptide composition, assembly and phosphorylation patterns of the Photosystem II antenna system of Chlamydomonas reinhardtii. Planta 194: 42–54
Anastassiou R and Argyroudi-Akoyunoglou JH (1995) Thylakoid-bound proteolytic activity against LHC II apoprotein in bean. Photosynth Res 43: 241–250
Auchincloss AH, Alexander A and Kohorn BD (1992) Requirement for three membrane-spanning α-helices in the post-translational insertion of a thylakoid membrane protein. J Biol Chem 267: 10439–10446
Baillet B and Korhorn BD (1996) Hydrophobic core but not amino-terminal charged residues are required for translocation of an integral thylakoid membrane protein in vivo. J Biol Chem 271: 18375–18378
Barber J, Nield J, Morris EP and Hankamer B (1999) Subunit positioning in Photosystem II revisited. Trends Biochem Sci 24: 43–45
Bassi R and Wollman F-A (1991) The chlorophyll-a/b proteins of Photosystem II in Chlamydomonas reinhardtii. Planta 183: 423–433
Bassi R, Sandonà D and Croce R (1997) Novel aspects of chlorophyll a/b-binding proteins. Physiol Plant 100: 769–779
Bellemare G, Bartlett SG and Chua N-H (1982) Biosynthesis of chlorophyll a/b-binding polypeptides in wild type and the chlorina f2 mutant of barley. J Biol Chem 257: 7762–7767
Belyaeva O and Sundqvist C (1998) Comparative investigation of the appearance of primary chlorophyllide forms in etiolated leaves, prolamellar bodies and prothylakoids. Photosynth Res 55: 41–48
Bennett J (1981) Biosynthesis of the light-harvesting chlorophyll a/b protein. Peptide turnover in darkness. Eur J Biochem 118: 61–70
Bonk M, Hoffmann B, Von Lintig J, Schledz M, Al-Babili S, Hobeika E, Kleinig H and Beyer P (1997) Chloroplast import of four carotenoid biosynthetic enzymes in vitro reveals differential fates prior to membrane binding and oligomeric assembly. Eur J Biochem 247: 942–950
Booth PJ and Paulsen H (1996) Assembly of light-harvesting chlorophyll a/b complex in vitro. Time-resolved fluorescence measurements. Biochemistry 35: 5103–5108
Cammarata KV and Schmidt GW (1992) In vitro reconstitution of a light-harvesting gene product: deletion mutagenesis and analysis of pigment binding. Biochemistry 31: 2779–2789
Chitnis PR, Harel E, Kohorn BD, Tobin EM and Thornber JP (1986) Assembly of the precursor and processed light-harvesting chlorophyll a/b protein of Lemna into the light-harvesting complex II of barley etioplasts. J Cell Biol 102: 982–988
Christopher DA and Mullet JE (1994) Separate photosensory pathways co-regulate blue light/ultraviolet-A-activated psbD–psbC transcription and light-induced D2 and CP43 degradation in barley (Hordeum vulgare) chloroplasts. Plant Physiol 104: 1119–1129
Chunaev AS, Mirnaya ON, Maslov VG and Boschetti A (1991) Chlorophyll b-and loroxanthin-deficient mutants of Chlamydomonas reinhardtii. Photosynthetica 25: 291–301
Cline K (1986) Import of proteins into chloroplasts: membrane integration of a thylakoid precursor protein reconstituted in chloroplast lysates. J Biol Chem 261: 14804–14810
Cline K (1988) Light-harvesting chlorophyll a/b protein. Membrane insertion, proteolytic processing, assembly into LHC II, and localization to appressed membranes occurs in chloroplast lysates. Plant Physiol 86: 1120–1126
Cline K and Henry R (1996) Import and routing of nucleus-encoded chloroplast proteins. Annu Rev Cell Devel Biol 12: 1–26
Cline K, Henry R, Li C and Yuan J (1993) Multiple pathways for protein transport into or across the thylakoid membrane. EMBO J 12: 4105–4114
Cline K, Fulsom, DR and Viitanen PV (1989) An imported thylakoid protein accumulates in the stroma when insertion into thylakoids is inhibited. J Biol Chem 264: 14225–14232
Connelly JP, Müller MG, Bassi R, Croce R and Holzwarth AR (1997) Femtosecond transient absorption study of carotenoid to chlorophyll energy transfer in the light-harvesting complex II of Photosystem II. Biochemistry 36: 281–288
Cuming AC and Bennett J (1981) Biosynthesis of the light-harvsting chlorophyll a/b protein. Control of messenger RNA activity by light. Eur J Biochem 118: 71–80
Dahlin C and Cline K (1991) Developmental regulation of the plastid protein import apparatus. Plant Cell 3: 1131–1140
Dreyfuss BW and Thornber JP (1994) Assembly of the lightharvesting complexes (LHCs) of Photosystem II. Plant Physiol 106: 829–839
Eggink LL, Park H and Hoober JK (1999) The role of the envelope in assembly of light-harvesting complexes in the chloroplast: distribution of LHCP between chloroplast and vacuoles during chloroplast development in Chlamydomonas reinhardtii. In: Argyroudi-Akoyunoglou JH and Senger H (eds) The Chloroplast: From Molecular Biology to Biotechnology, pp 161–166. Kluwer Academic Publishers, Dordrecht, The Netherlands
Eichenberger W, Boschetti A and Michel HP (1986) Lipid and pigment composition of a chlorophyll b-deficient mutant of Chlamydomonas reinhardtii. Physiol Plant 66: 589–594
Eullaffroy P, Popovic R and Frank F (1998) Changes of chlorophyll(ide) fluorescence yield induced by a short light pulse as a probe to monitor the early steps of etioplast phototransformation in dark-grown leaves. Photochem Photobiol 67: 676–682
Falbel TG, Meehl JB and Staehelin LA (1996) Severity of mutant phenotype in a series of chlorophyll-deficient wheat mutants depends on light intensity and the severity of the block in chlorophyll synthesis. Plant Physiol 112: 821–832
Flachmann R and Kühlbrandt W (1996) Crystallization and identification of an assembly defect of recombinant antenna complexes produced in transgenic tobacco plants. Proc Natl Acad Sci USA 93: 14966–14971
Ford C and Wang W-Y. (1980) Three new yellow loci in Chlamydomonas reinhardtii. Mol Gen Genet 179: 259–263
Frank HA and Cogdell RJ (1996) Carotenoids in photosynthesis. Photochem Photobiol 63: 257–264
Franklin AE and Hoffman NE (1993) Characterization of a chloroplast homologue of the 54-kDa subunit of the signal recognition particle. J Biol Chem 268: 22175–22180
Friedman AL and Keegstra K (1989) Chloroplast protein import. Quantitative analysis of precursor binding. Plant Physiol 89: 993–999
Frydman J, Nimmesgern E, Ohtsuka K and Hartl FU (1994) Folding of nascent polypeptide chains in a high molecular mass assembly with molecular chaperones. Nature 370: 111–117
Funk C, Adamska I, Green BR, Andersson B and Renger G (1995) The nuclear-encoded chlorophyll-binding Photosystem II-S protein is stable in the absence of pigments. J Biol Chem 270: 30141–30147
Funk C, Lindström V and Vermaas W (1999) Small Cab-like proteins: relatives to the chlorophyll a/b binding proteins in cyanobacteria. In: Argyroudi-Akoyunoglou JH and Senger H (eds) The Chloroplast: From Molecular Biology to Biotechnology, pp 103–106. Kluwer Academic Publishers, Dordrecht, The Netherlands
Guiffra E, Cugini D, Croce R and Bassi R (1996) Reconstitution and pigment-binding properties of recombinant CP29. Eur J Biochem 238: 112–120
Giuffra E, Zucchelli G, Sandonà D, Croce R, Cugini D, Garlaschi FM, Bassi R and Jennings RC (1997) Analysis of some optical properties of a native and reconstituted Photosystem II antenna complex, CP29: Pigment binding sites can be occupied by chlorophyll a or chlorophyll b and determine spectral forms. Biochemistry 36: 12984–12993
Gradinaru CC, Özdemir S, Gülen D, Van Stokkum IHM, Van Grondelle R and van Amerongen H (1998) The flow of excitation energy in LHC II monomers: Implications for the structural model of the major plant antenna. Biophys J 75: 3064–3077
Green BR and Kühlbrandt W (1995) Sequence conservation of light-harvesting and stress-response proteins in relation to the three-dimensional molecular structure of LHC II. Photosynth Res 44: 139–148
Green BR and Pichersky E (1994) Hypothesis for the evolution of three-helix Chl a/b and Chl a/c light-harvesting antenna proteins from two-helix and four-helix ancestors. Photosynth Res 39: 149–162
Grimm B, Kruse E and Kloppstech K (1989) Transiently expressed early light-inducible proteins share transmembrane domains with light-harvesting chlorophyll binding proteins. Plant Mol Biol 13: 583–593
Hahn D and Kück U (1999) Identification of DNA sequences controlling light-and chloroplast-dependent expression of the lhcb1 gene from Chlamydomonas reinhardtii. Curr Genet 34: 459–466
Heinze I, Pfündel E, Hühn M and Dau H (1997) Assembly of light harvesting complexes II (LHC II) in the absence of lutein. A study on the α-carotenoid-free mutant C-2′-34 of the green alga Scenedesmus obliquus. Biochim Biophys Acta 1320: 188–194
Hemelrijk PW, Kwa SLS, Van Grondelle R and Dekker JP (1992) Spectroscopic properties of LHC II, the main light-harvesting chlorophyll a/b protein complex from chloroplast membranes. Biochim Biophys Acta 1098: 159–166
Hermsmeier D, Schulz R and Senger H (1994) Formation of light-harvesting complexes of Photosystem II in Scenedesmus. 1.Correlation between amounts of photosynthetic pigments, Lhc messenger RNAs and LHC apoproteins during constitutional dark and light-dependent Lhc-gene expression. Planta 193: 398–405
High S, Henry R, Mould RM, Valent Q, Meacock S, Cline K, Gray JC and Luirink J (1997) Chloroplast SRP54 interacts with a specific subset of thylakoid precursor proteins. J Biol Chem 272: 11622–11628
Hiller RG, Broughton MJ, Wrench PM, Sharples FP, Miller DJ and Catmull J (1999) Dinoflagellate light-harvesting proteins: genes, structure and reconstitution. In: Argyroudi-Akoyunoglou JH and Senger H (eds) The Chloroplast: From Molecular Biology to Biotechnology, pp 3–10. Kluwer Academic Publishers, Dordrecht, The Netherlands
Hobe S, Förster R, Klingler J and Paulsen H (1995) N-Proximal sequence motif in light-harvesting chlorophyll a/b-binding protein is essential for trimerization of light-harvesting chlorophyll a/b complex. Biochemistry 34: 10224–10228
Hoffman NE and Franklin AE (1994) Evidence for a stromal GTP requirement for the integration of a chlorophyll a/b-binding polypeptide into thylakoid membranes. Plant Physiol 105: 295–304
Hoober JK and Hughes MJ (1992) Purification and characterization of a membrane-bound protease from Chlamydomonas reinhardtii. Plant Physiol 99: 932–937
Hoober JK, Boyd CO and Paavola LG (1991) Origin of thylakoid membranes in Chlamydomonas reinhardtii y-1 at 38 °C. Plant Physiol 96: 1321–1328
Hoober JK, Marks DB, Keller BJ and Margulies MM (1982) Regulation of accumulation of the major thylakoid polypeptides in Chlamydomonas reinhardtii y-1 at 25 °C and 38 °C. J Cell Biol 95: 552–558
Hoober JK, Maloney MA, Asbury LR and Marks DB (1990) Accumulation of chlorophyll a/b-binding polypeptides in Chlamydomonas reinhardtii y-1 in the light or dark at 38 °C. Plant Physiol 92: 419–426
Hoober JK, White RA, Marks DB and Gabriel JL (1994) Biogenesis of thylakoid membranes with emphasis on the process in Chlamydomonas. Photosynth Res 39: 15–31
Hoober JK, Park H, Wolfe GR, Komine Y and Eggink LL (1998) Assembly of light-harvesting systems. In: Rochaix J-D, Goldschmidt-Clermont M and Merchant S (eds) The Molecular Biology of Chloroplasts and Mitochondria in Chlamydomonas, pp 363–376. Kluwer Academic Publishers, Dordrecht, The Netherlands
Horton P, Ruban AV and Walters RG (1996) Regulation of light harvesting in green plants. Annu Rev Plant Physiol Plant Mol Biol 47: 655–684
Ide JP, Klug DR, Kühlbrandt W, Giorgi LB and Porter G (1987) The state of detergent solubilized light-harvesting chlorophyll a/b protein complex as monitored by picosecond time-resolved fluorescence and circular dichroism. Biochim Biophys Acta 893: 349–364
Jakobsson E (1997) Computer simulation studies of biological membranes: progress, promise and pitfalls. Trends Biochem Sci 22: 339–344
Jansen MAK, Mattoo AK and Edelman M (1999) D1–D2 protein degradation in the chloroplast. Complex light saturation kinetics. Eur J Biochem 260: 527–532
Jansson S (1994) The light-harvesting chlorophyll a/b-binding proteins. Biochim Biophys Acta 1184: 1–19
Jansson S (1999) A guide to the Lhc genes and their relatives in Arabidopsis. Trends Plant Sci 4: 236–240
Joyard J, Block MA and Douce R (1991) Molecular aspects of plastid envelope biochemistry. Eur J Biochem 199: 489–509
Joyard J, Maréchal E, Miège C, Block MA, Dorne A-J and Douce R (1998) Structure, distribution and biosynthesis of glycerolipids from higher plant chloroplasts. In: Siegenthaler P-A and Murata N (eds) Lipids in Photosynthesis: Structure, Function and Genetics, pp 21–52. Kluwer Academic Publishers, Dordrecht, The Netherlands
Katz JJ, Dougherty RC and Boucher LJ (1966) Infrared and nuclear magnetic resonance spectroscopy of chlorophyll. In: Vernon LP and Seely GR (eds) The Chlorophylls, pp 185–251. Academic Press, New York
Keegstra K and Cline K (1999) Protein import and routing systems in chloroplasts. Plant Cell 11: 557–570
Kim SJ, Jansson S, Hoffman NE, Robinson C and Mant A (1999) Distinct ‘assisted’ and 'spontaneous’ mechanisms for the insertion of polytopic chlorophyll-binding proteins into the thylakoid membrane. J Biol Chem 274: 4715–4721
Klimyuk VI, Persello-Cartieaux F, Havaux M, Contard-David P, Schuenemann D, Meiherhoff K, Gouet P, Jones JDG, Hoffman NE and Nussaume L (1999) A chromodomain protein encoded by the Arabidopsis CAO gene is a plant-specific component of the chloroplast signal recognition particle pathway that is involved in LHCP targeting. Plant Cell 11: 87–99
Kohorn BD (1990) Replacement of histidines of light harvesting chlorophyll a/b binding protein II disrupts chlorophyll–protein complex assembly. Plant Physiol 93: 339–342
Kohorn BD and Auchincloss AH (1991) Integration of a chlorophyll-binding protein into Escherichia coli membranes in the absence of chlorophyll. J Biol Chem 266: 12048–12052
Kohorn BD and Tobin EM (1987) Amino acid charge distribution influences the assembly of apoprotein into light-harvesting complex II. J Biol Chem 262: 12897–12899
Kohorn BD, Harel E, Chitnis PR, Thornber JP and Tobin EM (1986) Functional and mutational analysis of the light-harvesting chlorophyll a/b protein of thylakoid membranes. J Cell Biol 102: 972–981
Komine Y, Park H, Wolfe GR and Hoober JK (1996) Secretory granules in the cytoplasm of a wall-less mutant of Chlamydomonas reinhardtii contain processed light-harvesting complex apoproteins and HSP70. J Photochem Photobiol B: Biol 36: 301–306
Kouranov A, Chen X, Fuks B and Schnell DJ (1998) Tic20 and Tic22 are new components of the protein import apparatus at the chloroplast inner envelope membrane. J Cell Biol 143: 991–1002
Kühlbrandt W and Wang DN (1991) Three-dimensional structure of plant light-harvesting complex determined by electron crystallography. Nature 350: 130–134
Kühlbrandt W, Wang DN and Fujiyoshi Y (1994) Atomic model of plant light-harvesting complex by electron crystallography. Nature 367: 614–621
Kuttkat A, Edhofer I, Eichacker LA and Paulsen H (1997) Light-harvesting chlorophyll a/b-binding protein stably inserts into etioplast membranes supplemented with Zn-pheophytin a/b. J Biol Chem 272: 20451–20455
Kuttkat A, Grimm R and Paulsen H (1995) Light-harvesting chlorophyll a/b-binding protein inserted into isolated thylakoids binds pigments and is assembled into trimeric light-harvesting complex. Plant Physiol 109: 1267–1276
Kuttkat A, Hartmann A, Hobe S and Paulsen H (1996) The C-terminal domain of light-harvesting chlorophyll-a/b-binding protein is involved in the stabilization of trimeric light-harvesting complex. Eur J Biochem 242: 288–292
Li X, Henry R, Yuan J, Cline K and Hoffman NE (1995) A chloroplast homologue of the signal recognition particle subunit SRP54 is involved in the posttranslational integration of aprotein into thylakoid membranes. Proc Natl Acad Sci USA 92: 3789–3793
Lindahl M, Yang D-H and Andersson B (1995) Regulatory proteolysis of the major light-harvesting chlorophyll a/b protein of Photosystem II by a light-induced membrane-associated enzymic system. Eur J Biochem 231: 503–509
Maloney MA, Hoober JK and Marks DB (1989) Kinetics of chlorophyll accumulation and formation of chlorophyll–protein complexes during greening of Chlamydomonas reinhardtii y1 at 38 °C. Plant Physiol 91: 1100–1106
Maréchal E, Block MA, Dorne A-J, Douce R and Joyard J (1997) Lipid synthesis and metabolism in the plastid envelope. Physiol Plant 100: 65–77
Marks DB, Keller BJ and Hoober JK (1985) In vitro processing of precursors of thylakoid membrane proteins of Chlamydomonas reinhardtii y-1. Plant Physiol 79: 108–113
Matringe M, Camadro J-M, Block MA, Joyard J, Scalla R, Lsabbe P and Douce R (1992) Localization within chloroplasts of protoporphyrinogen oxidase, the target enzyme for diphenylether-like herbicides. J Biol Chem 267: 4646–4651
Meyer G and Kloppstech K (1984) A rapidly light-induced chloroplast protein with a high turnover coded for by pea nuclear DNA. Eur J Biochem 138: 201–207
Michel H, Tellenbach M and Boschetti A (1983) A chlorophyll b-less mutant of Chlamydomonas reinhardtii lacking in the light-harvesting chlorophyll a/b–protein complex but not in its apoproteins. Biochim Biophys Acta 725: 417–424
Morré DJ, Penel C, Morré DM, Sandelius AS, Moreau P and Andersson B (1991a) Cell-free transfer and sorting of membrane lipids in spinach. Donor and acceptor specificity. Protoplasma 160: 49–64
Morré DJ, Selldén G, Sundqvist C and Sandelius AS (1991b) Stromal low temperature compartment derived from the inner membrane of the chloroplast envelope. Plant Physiol 97: 1558–1564
Murray DL and Kohorn BD (1991) Chloroplasts of Arabidopsis thaliana homozygous for the ch-1 locus lack chlorophyll b, lack stable LHCP II and have stacked thylakoids. Plant Mol Biol 16: 71–79
Ohad I, Siekevitz P and Palade GE (1967) Biogenesis of chloroplast membranes. II. Plastid differentiation during greening of a dark-grown algal mutant (Chlamydomonas reinhardi). J Cell Biol 35: 553–584
Ohlrogge J and Browse J (1995) Lipid biosynthesis. Plant Cell 7: 957–970
Pagano A, Cinque G and Bassi R (1998) In vitro reconstitution of the recombinant Photosystem II light-harvesting complex CP24 and its spectroscopic characterization. J Biol Chem 273: 17154–17165
Park H, Eggink LL, Roberson RW and Hoober JK (1999) Transfer of proteins from the chloroplast to vacuoles in Chlamydomonas reinhardtii (Chlorophyta): a pathway fordegradation. J Phycol 35: 528–538
Park H and Hoober JK (1997) Chlorophyll synthesis modulates retention of apoproteins of light-harvesting complex II by the chloroplast in Chlamydomonas reinhardtii. Physiol Plant 101: 135–142
Parks GD and Lamb RA (1993) Role of NH2-terminal positively charged residues in establishing membrane protein topology. J Biol Chem 268: 19101–19109
Paulsen H (1997) Pigment ligation to proteins of the photosynthetic apparatus in higher plants. Physiol Plant 100: 760–768
Paulsen H, Finkenzeller B and Kühlein N (1993) Pigments induce folding of light-harvesting chlorophyll a/b-binding protein. Eur J Biochem 215: 809–816
Peter GF and Thornber JP (1991) Biochemical composition and organization of higher plant Photosystem II light-harvesting pigment–proteins. J Biol Chem 266: 16745–16754
Pilgrim MP, Van Wijk K-J, Parry DH, Sy DAC and Hoffman N (1998) Expression of a dominant negative form of cpSRP54 inhibits chloroplast biogenesis in Arabidopsis. Plant J 13: 177–186
Plumley FG and Schmidt GW (1995) Light-harvesting chlorophyll a/b complexes: interdependent pigment synthesis and protein assembly. Plant Cell 7: 689–704
Rawyler A, Meylan-Bettex M and Siegenthaler PA (1995) (Galacto)lipid export from envelope to thylakoid membranes in intact chloroplasts. II. A general process with a key role for the envelope in the establishment of lipid asymmetry in thylakoid membranes. Biochim Biophys Acta 1233: 123–133
Reed JE, Cline K, Stephens LC, Bacot KO and Viitanen PV (1990) Early events in the import/assembly pathway of an integral thylakoid protein. Eur J Biochem 194: 33–42
Reinero A and Tobin EM (1991) An amino-proximal hydrophobic domain in the major light-harvesting chlorophyll a/b–protein is essential for membrane integration and protein stability. Photosynth Res 30: 25–33
Reinbothe C, Lebedev N, Apel K and Reinbothe S (1997) Regulation of chloroplast protein import through a protochlorophyllide-responsive transit peptide. Proc Natl Acad Sci USA 94: 8890–8894
Renge I and Avarmaa R (1985) Specific solvation of chlorophyll a: solvent nucleophilicity, hydrogen bonding and steric effects on absorption spectra. Photochem Photobiol 42: 253–260
Rhee K-H, Morris EP, Barber J and Kühlbrandt W (1998) Three-dimensional structure of the plant Photosystem II reaction center at 8 Å resolution. Nature 396: 283–286
Richter S and Lamppa GK (1998) A chloroplast processing enzyme functions as the general stromal processing peptidase. Proc Natl Acad Sci USA 95: 7463–7468
Ruban AV, Phillip D, Young AJ and Horton P (1998) Excited-state energy level does not determine the differential effect of violaxanthin and zeaxanthin on chlorophyll fluorescence quenching in the isolated light-harvesting complex of Photosystem II. Photochem Photobiol 68: 829–834
Ryberg M and Sundqvist C (1988) The regular ultrastructure of isolated prolamellar bodies depends on the presence of membrane-bound NADPH-protochlorophyllide oxidoreductase. Physiol Plant 73: 218–226
Sager R (1955) Inheritance in the green alga Chlamydomonas reinhardi. Genetics 40: 476–489
Sandelius AS and Selstam E (1984) Localization of galactolipid biosynthesis in etioplasts isolated from dark-grown wheat (Triticum aestivum L.). Plant Physiol 76: 1041–1046
Sandonà D, Croce R, Pagano A, Crimi M and Bassi R (1998) Higher plant light harvesting proteins. Structure and function as revealed by mutation analysis of either protein or chromophore moieties. Biochim Biophys Acta 1365: 207–214
Sato N, Tsuzuki M, Matsuda Y, Ehara T, Osafune T and Kawaguchi A (1995) Isolation and characterization of mutants affected in lipid metabolism of Chlamydomonas reinhardtii. Eur J Biochem 230: 987–993
Schnell DJ and Blobel G (1993) Identification of intermediates in the pathway of protein import into chloroplasts and their localization to envelope contact sites. J Cell Biol 120: 103–115
Schuenemann D, Gupta S, Persello-Cartieaux F, Klimyuk VI, Jones JDG, Nussaume L and Hoffman NE (1998) A novel signal recognition particle targets light-harvesting proteins to the thylakoid membranes. Proc Natl Acad Sci USA 95: 10312–10316
Searle G, Brody SS and Van Hoek A (1990) Evidence for the formation of a chlorophyll a/zeaxanthin complex in lecithin liposomes from fluorescence decay kinetics. Photochem Photobiol 52: 401–407
Selstam E (1998) Development of thylakoid membranes with respect to lipids. In: Siegenthaler P-A and Murata N (eds) Lipids in Photosynthesis: Structure, Function and Genetics, pp 209–224. Kluwer Academic Publishers, Dordrecht, The Netherlands
Selstam E and Sandelius AS (1984) A comparison between prolamellar bodies and prothylakoid membranes of etioplasts of dark-grown wheat concerning lipid and polypeptide composition. Plant Physiol 76: 1036–1040
Siefermann-Harms D (1985) Carotenoids in photosynthesis. I. Location in photosynthetic membranes and light-harvesting function. Biochim Biophys Acta 811: 325–355
Sigrist M and Staehelin LA (1994) Appearance of type 1, 2 and 3 light-harvesting complex II and light-harvesting complex I proteins during light-induced greening of barley (Hordeum vulgare) etioplasts. Plant Physiol 104: 135–145
Sigrist M, Zwillenberg C, Giroud C, Eichenberger W and Boschetti A (1988) Sulfolipid associated with the light-harvesting complex associated with Photosystem II apoproteins of Chlamydomonas reinhardtii. Plant Sci 58: 15–23
Silva-Filho MC, Wieërs M-C, Flügge U-I, Chaumont F and Boutry M (1997) Different in vitro and in vivo targeting properties of the transit peptide of a chloroplast envelope inner membrane protein. J Biol Chem 272: 15264–15269
Soll J and Tien R (1998) Protein translocation into and across the chloroplast envelope membranes. Plant Mol Biol 38: 191–207
Smith TA and Kohorn BD (1994) Mutations in a signal sequence for the thylakoid membrane identify multiple protein transport pathways and nuclear suppressors. J Cell Biol 126: 365–374
Sundqvist C and Dahlin C (1997) With chlorophyll pigments from prolamellar bodies to light-harvesting complexes. Physiol Plant 100: 748–759
Tanaka A, Ito H, Tanaka R, Tanaka NK Yoshida K and Okada K (1998) Chlorophyll a oxygenase (CAO) is involved in chlorophyll b formation from chlorophyll a. Proc Natl Acad Sci USA 95: 12719–12723
Terao T and Katoh S (1989) Synthesis and breakdown of the apoproteins of light-harvesting chlorophyll a/b proteins in chlorophyll b-deficient mutants of rice. Plant Cell Physiol 30: 571–580
Thornber JP and Highkin HR (1974) Composition of the photosynthetic apparatus of normal barley leaves and a mutant lacking chlorophyll b. Eur J Biochem 41: 109–116
Timko MP (1998) Pigment biosynthesis: chlorophlls, heme and carotenoids. In: Rochaix J-D, Goldschmidt-Clermont M and Merchant S (eds) The Molecular Biology of Chloroplasts and Mitochondria in Chlamydomonas, pp 377–414. Kluwer Academic Publishers, Dordrecht, The Netherlands
Von Heijne G (1994) Membrane proteins: from sequence to structure. Annu Rev Biophys Biomol Struct 23: 167–192
Von Wettstein D, Gough S and Kannangara CG (1995) Chlorophyll biosynthesis. Plant Cell 7: 1039–1057
Waegemann K, Paulsen H and Soll J (1990) Translocation of proteins into isolated chloroplasts requires cytosolic factors to obtain import competence. FEBS Lett 261: 89–92
White RA and Hoober JK (1994) Biogenesis of thylakoid membranes in Chlamydomonas reinhardtii y1. Plant Physiol 106: 583–590
White RA, Wolfe GR, Komine Y and Hoober JK (1996) Localization of light-harvesting complex apoproteins in the chloroplast and cytoplasm during greening of Chlamydomonas reinhardtii at 38 °C. Photosynth Res 47: 267–280
Wolfe GR, Park H, Sharp WP and Hoober JK (1997) Light-harvesting complex apoproteins in cytoplasmic vacuoles in Chlamydomonas reinhardtii (Chlorophyta). J Phycol 33: 377–386
Yang D-H, Webster J, Adam Z, Lindahl M and Andersson B (1998) Induction of acclimative proteolysis of the light-harvesting chlorophyll a/b protein of Photosystem II in response to elevated light intensities. Plant Physiol 118: 827–834
Yuan J, Cline K and Theg SM (1991) Cryopreservation of chloroplasts and thylakoids for studies of protein import and integration. Plant Physiol 95: 1259–1264
Yuan J, Henry R and Cline K (1993) Stromal factor plays an essential role in protein integration into thylakoids that cannot be replaced by unfolding or by heat shock protein Hsp70. Proc Natl Acad Sci USA 90: 8552–8556
Rights and permissions
About this article
Cite this article
Kenneth Hoober, J., Eggink, L.L. Assembly of light-harvesting complex II and biogenesis of thylakoid membranes in chloroplasts. Photosynthesis Research 61, 197–215 (1999). https://doi.org/10.1023/A:1006313703640
Issue Date:
DOI: https://doi.org/10.1023/A:1006313703640