Abstract
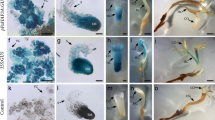
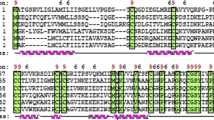
A full-length Picea abies cDNA clone Pa18, encoding a protein with the characteristics of plant lipid transfer proteins, has been isolated and characterized. The size of the deduced 173 amino acid (aa) long protein is around 18 kDa. The first 100–120 aa show similarity to angiosperm lipid transfer proteins in amino acid sequence as well as in predicted secondary structure. The Pa18 gene is constitutively expressed in embryogenic cultures of Picea abies representing different stages of development as well as in non-embryogenic callus and seedlings. The Pa18 gene product has an antimicrobial activity. In situ hybridization showed that the Pa18 gene is equally expressed in all embryonic cells of proliferating embryogenic cultures but during embryo maturation the expression of the gene in maturing and mature somatic as well as in mature zygotic embryos is stronger in the outer cell layer than in other tissues. Southern blot analysis at different stringencies was consistent with a single gene with one or two copies rather than a gene family. Twenty independent transgenic sublines over- and under-expressing the Pa18 gene under the Zea mays ubiquitin promoter were established. There was a high yield of mature somatic embryos with a smooth surface only in untransformed, control cultures. Irrespective of the expression level of Pa18, the somatic embryos started to mature when given a maturation treatment. However, in the transgenic sublines, the outer cells in the maturing embryos frequently became elongated and vacuolated instead of remaining small and uniform. One explanation for this was that the expression of Pa18 was not restricted to the outer cell layer in transformed sublines. Angiosperms and gymnosperms separated about 300 million years ago and the embryo genesis is different in the two groups. The outer cell layer (protoderm), the first tissue to differentiate, is less clearly delineated in gymnosperms. For normal embryo development in angiosperms, expression of the LTP gene must be restricted to the protodermal cells. In this work we show that the expression of the Pa18 gene must be restricted to the putative protodermal cells of the gymnosperm.
Similar content being viewed by others
References
Arondel, V. and Kader, J.-C., 1990. Lipid transfer in plants. Experientia 46: 579–585.
Baldan B., Guzzo, F., Filippini, F., Gasparian, M., LoSchiavo, F., Vitale, A., de Vries, S.C, Mariani, P. and Terzi, M. 1997. The secretory nature of the lesion of carrot cell variant ts11, rescuable by endochitinases. Planta 203: 381–389.
Bernatzky, R. and Tanksley, S.D. 1986. Genetics of actin-related sequences in tomato. Theor. Appl. Genet. 73: 314–321.
Bernhard, W.R., Thoma, S., Botella, J. and Somerville C. 1991. Isolation of a cDNA clone for spinach lipid transfer protein and evidence that the protein is synthesized by the secretory pathway. Plant Physiol. 95: 164–170.
Bozhkov, P.V. and von Arnold, S. 1998. Polyethylene glycol promotes maturation but inhibits further development of Picea abies somatic embryos. Physiol. Plant. 104: 211–224.
Caaveiro, J.M.M., Molina, A., González-Manas, J.M., Rodríguez-Palenzuela, P., Garcia-Olmedo, F. and Goni, F.M. 1997. Differential effects of five types of antipathogenic plant peptides on model membranes. FEBS Lett. 410: 338–342.
Cammue, B.P.A., Thevissen, K., Hendriks, M., Eggermont, K., Goderis, I.J., Proost, P., Van Damme, J., Osborn, R.W., Guerbette, F., Kader, J-C. and Broekaert, W.F. 1995. A potent antimicrobial protein from onion seeds showing sequence homology to plant lipid transfer proteins. Plant Physiol. 109: 445–455.
Canevascini, S., Caderas, D., Mandel, T., Fleming, A.J., Dupuis, I. and Kuhlemeier, C. 1996. Tissue-specific expression and promoter analysis of the tobacco ltp1 gene. Plant Physiol. 112: 513–524.
Choi, D-W., Song, J.Y., Oh, M.-H., Lee, J.S., Moon, J., Suh, S. and Kim, S.-G. 1996. Isolation of a root-specific cDNA encoding a ns-LTP-like protein from the roots of bean (Phaseolus vulgaris L.) seedlings. Plant Mol. Biol. 30: 1059–1066.
Christensen, A.H. and Quail, P.H. 1996. Ubiquitin promoter-based vectors for high-level expression of selectable and/or screenable marker genes in monocotyledonous plants. Transgen. Res. 5: 213–218.
Clapham, D., Manders, G., Yibrah, H.S. and von Arnold, S. 1995. Enhancement of short-and medium-term expression of transgenes in embryogenic suspensions of Picea abies (L.) Karst. J. Exp. Bot. 46: 655–662.
Clapham, D., Demel, P., Elfstrand, M., Koop, H.-U., Sabala, I. and von Arnold, S. 1999. Gene transfer by particle bombardment to embryogenic cultures and the production of transgenic plants. Scand. J. For. Res. (in press).
De Block, M. and Debrouwer, D. 1996. RNA-RNA in situ hybridization using DIG-labeled probes: the effect of high molecular weight polivinyl alcohol on the alkaline phosphatase indoxyl-nitroblue tetrazolium reaction. In: Nonradioactive In Situ Hybridization Application Manual, Boehringer Mannheim, Mannheim, Germany, pp. 141–145.
Désormeaux, A., Blochet, J.-E., Pézolet, M. and Marion, D. 1992. Amino acid sequence of a non-specific wheat phospholipid transfer protein and its conformation as revealed by infrared and Raman spectroscopy. Role of disulfide bridges and phospholipids in the stabilization of the α-helix structure. Biochim. Biophys. Acta 1121: 137–152.
Dodeman, V.L., Ducreux, G. and Kreis, M. 1997. Zygotic embryogenesis versus somatic embryogenesis. J. Exp. Bot. 48: 1493–1509.
Egertsdotter, U. and von Arnold, S. 1993. Classification of embryogenic cell-lines of Picea abies as regards protoplast isolation and culture. J. Plant Physiol. 141: 222–229.
Egertsdotter, U. and von Arnold, S. 1995. Importance of arabinogalactan proteins for the development of somatic embryos of Norway spruce (Picea abies). Physiol. Plant. 93: 334–345.
Filonova, L., Bozhkov, P.V. and von Arnold, S. 1998. Time-lapse tracking of origin and development of somatic embryos in a gymnosperm, Norway spruce. IX International Congress on Plant Tissue and Cell Culture, Jerusalem, Israel.
Finer, J.J., Vain, P., Jones, M.W. and McMullen, D.A. 1992. Development of the particle inflow gun for DNA delivery to plant cells. Plant Cell Rep. 11: 323–328.
Gallie, D.R. 1993. Posttranscriptional regulation of gene expression in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 44: 77–105.
García-Olmedo, F., Molina, A., Segura, A. and Moreno, M. 1995. The defensive role of nonspecific lipid-transfer proteins in plants. Trends Microbiol. 3: 72–74.
Gausing, K. 1994. Lipid transfer protein genes specifically expressed in barley leaves and coleoptiles. Planta 192: 574–580.
Gomar, J., Petit, M.-C., Sodano, P., Sy, D., Marion, D., Kader, J.-C., Vovelle, F. and Ptak, M. 1996. Solution structure and lipid binding of a nonspecific lipid transfer protein extracted from maize seeds. Protein Sci. 5: 565–577 (1996).
Hollenbach, B., Schreibe, L., Hartung, W. and Dietz, K.-J. 1997. Cadmium leads to stimulated expression of the lipid transfer protein genes in barley: implications for the involvement of lipid transfer proteins in wax assembly. Planta 203: 9–19.
Hughes, M.A., Dunn, M.A., Pearce, R.S., White, A.J. and Zhang, L. 1992. An abscisic acid-responsive, low temperature barley gene has homology with a maize phospholipid transfer protein. Plant Cell Env. 15: 861–865.
Jalonen, P. and von Arnold, S. 1991. Characterization of embryogenic cell lines of Picea abies in relation to their competence for maturation. Plant Cell Rep. 10: 384–387.
Jürgens, G., Mayer, U., Torres Ruiz, R.A., Berleth, T. and Miséra, S. 1991. Genetic analysis of pattern formation in the Arabidopsis thaliana. Development 1: 27–38.
Kader, J.-C. 1996. Lipid-transfer proteins in plants. Annu. Rev. Plant. Physiol. Plant Mol. Biol. 47: 627–654.
Keresztessy, Z. and Hughes, A. 1998. Homology modeling and molecular dynamics aided analysis of ligand complexes demonstrates functional properties of lipid-transfer proteins encoded by the barley low-temperature-inducible gene family, blt4. Plant J. 14: 522–533.
Kinlow, C.S., Gerttula, S.M. and Carter, M.C. 1994. Lipid transfer protein genes of loblolly pine are members of a complex gene family. Plant Mol. Biol. 26: 1213–1216.
Kyte, J. and Doolittle, R.F. 1982. A simple method for displaying the hydrophatic character of a protein. J. Mol. Biol. 157: 105–132.
Molina, A. and Garcia-Olmedo, F. 1997. Enhanced tolerance to bacterial pathogens caused by the transgenic expression of barley lipid transfer protein LTP2. Plant J. 12: 669–675.
Molina A., Diaz, I., Vasil, I.K., Carbonero, P. and García- Olmedo, F. 1996. Two cold-inducible genes encoding lipid transfer protein LTP4 from barley show differential responses to bacterial pathogens. Mol. Gen. Genet. 252: 162–168.
Mundy, J. and Rogers, J.C. 1986. Selective expression of a probable amylase/protease inhibitor in barley aleurone cells: comparison to the barley amylase/subtilisin inhibitor. Planta 169: 51–63.
Neumann, G.M., Condron, R., Svensson, B. and Polya, G.M. 1995. Purification, characterization and sequencing of a family of Petunia petal lipid transfer proteins phosphorylated by plant calcium-dependent protein kinase. Plant Sci. 107: 129–145.
Odani, S., Koide, T., Ono, T., Seto, Y. and Tanaka, T. 1987. Soybean hydrophobic protein. Isolation, partial characterization and the complete primary structure. Eur. J. Biochem. 162: 485–491.
Ostergaard, J., Hojrup, P. and Knudsen, J. 1995. Amino acid sequence of three acyl-binding/lipid-transfer proteins from rape seedlings. Biochim. Biophys. Acta 1254: 169–179.
Polya, G.M., Chandra, S., Chung, R., Neumann G.M. and Hoj, P.B. 1992. Purification and characterization of wheat and pine small basic protein substrates for plant calcium-dependent protein kinase. Biochim. Biophys. Acta 1120: 273–280.
Pyee, J. and Kolattukudy, P.E. 1995. The gene for the major cuticular wax-associated protein and three homologous genes from broccoli (Brassica oleracea) and their expression patterns. Plant J. 7: 49–59.
Sabala, I., Egertsdotter, U., von Fircks, H. and von Arnold, S. 1996. Abscisic acid-induced secretion of an antifreeze-like protein in embryogenic cell lines of Picea abies. J. Plant Physiol. 149: 163–170.
Sabala, I., Franzén, H. and von Arnold, S. 1997. A spruce gene, af70, constitutively expressed in somatic embryos and induced by ABA and low temperature in seedlings. Physiol. Plant. 99: 316–322.
Sambrook, J., Fritsch, E.F. and Maniatis, T.A. 1989. Molecular Cloning: A laboratory Manual, 2nd ed., Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
Saraste, M., Sibbald, P.R. and Wittinghofer, A. 1990. The P-loop a common motif in ATP-and GTP-binding proteins. Trends Biochem. Sci. 15: 430–434.
Singh, H. 1978. Embryology of gymnosperms. In: W. Zimmerman, Z. Carlquist, P. Ozeda and E.D. Wulff (Eds.) Handbuch der Pflanzenanatomie, Gebrüder Borntraeger, Berlin/Stuttgart, pp. 187–241.
Skriver, K., Leah, R., Müller-Uri, F., Olsen, F-L. and Mundy, J. 1992. Structure and expression of the barley lipid transfer protein gene Ltp1. Plant Mol. Biol. 18: 585–589.
Sossountzov, L., Ruiz-Avila, L., Vignols, F., Jolliot, A., Arondel, V., Tchang, F., Grosbois, M., Guerbette, F., Miginiac, E., Delseny, M., Puigdomè nech, P. and Kader, J.-C. 1991. Spatial and temporal expression of a maize lipid transfer protein gene. Plant Cell 3: 923–933.
Soufleri, I.A., Vergnolle, C., Miginiac, E. and Kader, J.-C. 1996. Germination specific lipid transfer protein cDNAs in Brassica napus L. Planta 199: 229–237.
Sterk, P., Booij, H., Schellekens, G.A., van Kammen, A. and de Vries, S.C. 1991. Cell-specific expression of the carrot EP2 lipid transfer protein gene. Plant Cell 3: 907–921.
Takishima, K., Watanabe, S., Yamada, M., Suga, T. and Mamiya, G. 1988. Amino acid sequences of two nonspecific lipid-transfer proteins from germinated castor bean. Eur. J. Biochem. 177: 241–249.
Terras, F.R.G., Goderis, I.J., Van Leuven, F., Vanderleyden, J., Cammue, B.P.A. and Broekaert, W.F. 1992. In vitro antifungal activity of a radish (Raphanus sativus L.) seed protein homologous to nonspecific lipid transfer proteins. Plant Physiol. 100: 1055–1058.
Thoma, S., Kaneko, Y. and Somerville, C. 1993. A non-specific lipid transfer protein from Arabidopsis is a cell wall protein. Plant J. 3: 427–436.
Thoma, S., Hecht, U., Kippers, A., Botella, J., de Vries, S. and Somerville C. 1994. Tissue-specific expression of a gene encoding a cell wall-localized lipid transfer protein from Arabidopsis. Plant Physiol. 105: 35–45.
Trevino, M.B. and O'Connell, M.A. 1998. Three droughtresponsive members of the nonspecific lipid-transfer protein gene family in Lycopersicon pennellii show different developmental patterns of expression. Plant Physiol. 116: 1461–1468.
von Arnold, S. 1987. Improved efficiency of somatic embryogenesis in mature embryos of Picea abies (L.) Karst. Plant Physiol. 128: 233–244.
von Arnold, S., Clapham, D., Egertsdotter, U., Ekberg, I., Mo, H. and Yibrah, H. 1995. Somatic embryogenesis in Norway spruce (Picea abies). In: Y.P.S. Bajaj (Ed.) Somatic Embryogenesis and Synthetic Seeds I, Springer-Verlag, Berlin/Heidelberg, pp. 415–430.
von Heijne, G. 1986. A new method for predicting signal sequence cleavage site. Nucl. Acid. Res. 14: 4683–4690.
Vroemen, C.W., Langeveld, S., Mayer, U., Ripper, G., Jürgens, G., van Kammen, A. and de Vries, S.C. 1996. Pattern formation in the Arabidopsis embryo revealed by position-specific lipid transfer protein gene expression. Plant Cell 8: 783–791.
Yadegari, R., de Paiva, G.R., Laux, T., Koltunow, A.M., Apuya, N., Zimmerman, J.L., Fischer, R.L., Harada, J.J. and Goldberg, R.B. 1994. Cell differentiation and morphogenesis are uncoupled in Arabidopsis raspberry embryos. Plant Cell 6: 1713–1729.
Yamada, M. 1992. Lipid transfer proteins in plants and microorganisms. Plant Cell Physiol. 33: 1–6.
Yibrah, H.S., Grönroos, R., Lindroth, A., Franzén, H., Clapham, D. and von Arnold, S. 1996. Agrobacterium rhizogenes-mediated induction of adventitious rooting from Pinus contorta hypocotyls and the effect of 5-azacytidine on transgene activity. Transgen. Res. 5: 75–85.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sabala, I., Elfstrand, M., Farbos, I. et al. Tissue-specific expression of Pa18, a putative lipid transfer protein gene, during embryo development in Norway spruce (Picea abies). Plant Mol Biol 42, 461–478 (2000). https://doi.org/10.1023/A:1006303702086
Issue Date:
DOI: https://doi.org/10.1023/A:1006303702086