Abstract
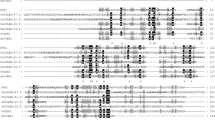
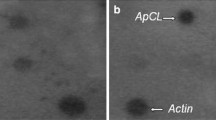
One of the major abiotic stresses that affect plant growth and development is anoxia or hypoxia. Plants respond to anoxia by regulation of gene expression at both the transcriptional and translational levels. Genes involved in such regulation are expected to be expressed soon after onset of anoxia. To date, however, anaerobically regulated genes that have been characterized predominantly encode enzymes for sugar phosphate metabolism, and are induced after several hours of anaerobic conditions. Early induced genes, those responding after 1–2 h of anoxia, have not been studied extensively. To study the early anaerobic response we investigated the most flooding-tolerant variety of rice, FR13A (flood-resistant). We used differential display techniques to identify cDNA fragments representing mRNAs that are induced within 90 min of anoxia. We isolated two cDNA fragments and one full-length cDNA that were induced to high levels. These cDNAs were found to be members of a family of 2–3 genes, which were called the aie (anaerobically inducible early) gene family. Northern blot analyses showed that the mRNA levels of aie genes peaked after 1.5 to 3 h of anoxia and were still at high levels after 72 h of anoxia. RNase protection assays showed 4–5 different protected bands indicating multiple transcripts from the aie gene family. Sequence analyses of the full-length cDNA showed an open reading frame that putatively encodes a 14 kDa protein of 127 amino acid residues. Neither the nucleotide nor the deduced amino acid sequences of this gene showed any significant homology to any known genes or proteins present in the GenBank or SwissProt databases. This novel gene, that is induced so early under anoxia in plants, may play an important role in plant metabolism under anaerobic conditions.
Similar content being viewed by others
References
Bailey-Serres, J. and Freeling, M. 1990. Hypoxic stress-induced changes in ribosomes of maize seedling roots. Plant Physiol. 94: 1237-1243.
Breviario, D., Morello, G.L. and Coraggio, I. 1994. Anaerobiosismediated early transcriptional and translational responses in rice (Oryza sativa L.) coleoptiles and roots. Plant Cell Environ. 17: 925-934.
Christopher, M.E. and Good, A.G. 1997. Effect of calcium and protein synthesis inhibitors on hypoxic induction of ldh and alaat. Plant Physiol. 114: 275.
Dennis, E.S., Gerlach, W.L., Pryor, A.J., Bennetzen, J.L., Inglis, A., Llewellyn, D., Sachs, M.M., Ferl, R.J. and Peacock, W.J. 1984. Molecular analysis of the alcohol dehydrogenase (adh1) gene of maize. Nucl. Acids Res. 12: 3983-4000.
Devereux, J., Haeberli, P. and Smithies, O. 1984. A comprehensive set of sequence analysis programs for the VAX. Nucl. Acids Res. 12: 387-395.
de Vetten, N.C. and Ferl, R.J. 1994. Transcriptional regulation of environmentally inducible genes in plants by an evolutionarily conserved family of G-box binding factors. Int. J. Biochem. 26: 1055-1068.
de Vetten, N.C. and Ferl, R.J. 1995. Characterization of a maize G-box binding factor that is induced by hypoxia. Plant J. 7: 589-601.
Dolferus, R., Jacobs, M., Peacock, W.J. and Dennis, E.S. 1994. Differential interactions of promoter elements in stress responses of the Arabidopsis adh1 gene. Plant Physiol. 105: 1075-1087.
Drew, M.C. 1997. Oxygen deficiency and root metabolism: injury and acclimation under hypoxia and anoxia. Annu. Rev. Plant Physiol. Plant Mol. Biol. 48: 223-250.
Drew, M.C., Jackson, M.B. and Giffard, S. 1979. Ethylenepromoted adventitious rooting and development of cortical air spaces (aerenchyma) in roots may be adaptive responses to flooding in Zea mays L. Planta 147: 83-88.
Ferl, R.J. 1990. ARF-B2: a protein complex that specifically binds to part of the anaerobic response element of maize adh1. Plant Physiol. 93: 1094-1101.
Giuliano, G., Pichersky, E., Malik, V.S., Timko, M.P., Scolnik, P.A. and Cashmore, A.R. 1988. An evolutionary conserved protein binding sequence upstream of a plant light-regulated gene. Proc. Natl. Acad. Sci. USA 85: 7089-7093.
Huq, E., Hossain, M.A. and Hodges, T.K. 1995. Cloning and sequencing of a cDNA encoding pyruvate decarboxylase 2 gene (accession No. U27350) from rice. Plant Physiol. 109: 722.
Jackson, M.B., Fenning, T.M., Drew, M.C. and Saker, L.R. 1985. Stimulation of ethylene production and gas-space (aerenchyma) formation in adventitious roots of Zea mays L. by small partial pressures of oxygen. Planta 165: 486-492.
Justin, S.H.F.W. and Armstrong, W. 1991. Evidence for the involvement of ethylene in aerenchyma formation in adventitious roots of rice. New Phytol. 118: 49-62.
Li, F., Barnathan, E.S. and Kariko, K. 1994. Rapid method for screening and cloning cDNAs generated in differential mRNA display: application of Northern blot for affinity capturing of cDNAs. Nucl. Acids Res. 22: 1764-1765.
Logemann, J., Schell, J. and Willmitzer, L. 1987. Improved method for the isolation of RNA from plant tissues. Anal Biochem. 163: 16-20.
Marck, C. 1988. DNA strider: a 'C' program for the fast analysis of DNA and protein sequences on the Apple Macintosh family of computers. Nucl. Acids Res. 16: 1829-1836.
McCouch, S.R., Kochert, G., Yu, Z.H., Wang, Z.Y., Khush, G.S., Coffman, W.R. and Tanksley, S.D. 1988. Molecular mapping of rice chromosomes. Theor. Appl. Genet. 76: 815-829.
Olive, M.R., Walker, J.C., Singh, K., Dennis, E.S. and Peacock, W.J. 1990. Functional properties of the anaerobic responsive element of the maize adh1 gene. Plant Mol. Biol. 15: 593-604.
Olive, M.R., Peacock, W.J. and Dennis, E.S. 1991. The anaerobic responsive elements contain two GC-rich sequences essential for binding a nuclear protein and hypoxic activation of the maize adh1 promoter. Nucl. Acids Res. 19: 7053-7060.
Paul, A.-L. and Ferl, R.J. 1997. The hypoxic response of three alcohol dehydrogenase genes: in vivo and in vitro footprinting of DNA/protein interactions describes multiple signalling connections. Ann. Bot. 79 (Suppl. A): 33-37.
Perata, P. and Alpi A. 1993. Plant responses to anaerobiosis. Plant Sci. 93: 1-17.
Ratcliffe, R.G. 1995. Metabolic aspects of the anoxic response in plant tissue. In: N. Smirnoff (ed.), Environment and Plant Metabolism, Bios Scientific Publishers, Oxford, pp. 111-127.
Ricard, B., Couee, I., Raymond, P., Saglio, P.H., Saint-Ges, V. and Pradet, A. 1994. Plant metabolism under hypoxia and anoxia. Plant Physiol. Biochem. 32: 1-10.
Rowland, L.J. and Strommer, J.N. 1986. Anaerobic treatment of maize root affects transcription of adh1 and transcript stability. Mol. Cell. Biol. 6: 3368-3372.
Russell, D.A. and Sachs, M.M. 1992. Protein synthesis in maize during anaerobic and heat stress. Plant Physiol. 99: 615-620.
Sachs, M.M., Freeling, M. and Okimoto, R. 1980. The anaerobic proteins of maize. Cell 20: 761-767.
Sachs, M.M., Subbaiah, C.C. and Saab, I.N. 1996. Anaerobic gene expression and flooding tolerance in maize. J. Exp. Bot. 47: 1-15.
Sambrook, J., Fritsch, E.F. and Maniatis, T. 1989. Molecular Cloning: A Laboratory Manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
Sheu, J.-J., Yu, T.-S., Tong, W.-F. and Yu, S.-M. 1996. Carbohydrate starvation stimulates differential expression of rice _-amylase genes that is modulated through complicated transcriptional and posttranscriptional processes. J. Biol. Chem. 271: 26998-27004.
Vayda, M.E., Shewmaker, C.K. and Morelli, J.K. 1995. Translational arrest in hypoxic potato tubers is correlated with the aberrant association of elongation factor EF-1_ with polysomes. Plant Mol. Biol. 28: 751-757.
Walker, J.C., Howard, E.A., Dennis, E.S. and Peacock, W.J. 1987. DNA sequences required for anaerobic expression of the maize alcohol dehydrogenase 1 gene. Proc. Natl. Acad. Sci. USA 84: 6624-6628.
Webster, C., Kim, C.-Y. and Roberts, J.K.M. 1991. Elongation and termination reactions of protein synthesis on maize root tip polyribosomes studied in a homologous cell-free systems. Plant Physiol. 96: 418-425.
Webster, C., Gaul, R.L., Browning, K.S., Ravel, J.M. and Roberts, J.K.M. 1992. Hypoxia enhances phosphorylation of eukaryotic initiation factor 4A in maize root tips. J. Biol. Chem. 266: 23341-23346.
Wenkert, W., Fausey, N.R. and Watters, H.D. 1981. Flooding responses in Zea mays L. Plant Soil 62: 351-366.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Huq, E., Hodges, T.K. An anaerobically inducible early (aie) gene family from rice. Plant Mol Biol 40, 591–601 (1999). https://doi.org/10.1023/A:1006284014613
Issue Date:
DOI: https://doi.org/10.1023/A:1006284014613