Abstract
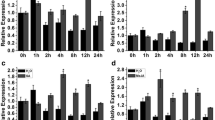
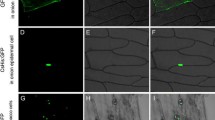
Many genes are induced by periods of water deficit, and a subset of these are dependent on elevated ABA content for expression. A number of drought-induced genes are not induced in leaves of the ABA-deficient mutant flacca from tomato (Lycopersicon esculentum) but are induced in detached, wilted wild-type leaves and ABA-treated leaves of both genotypes. The nucleotide sequence of the cDNA and corresponding genomic DNA fragment of one of these genes, his1-s (formerly called le20), encodes an amino acid sequence that is rich in Lys, Ala, and Ser. The predicted protein contains the tripartite structure of H1 histone and is similar to other H1 histones, especially in the globular domain. Since, his1-s is more closely related to a stress-induced gene from Lycopersicon pennellii than to another H1 histone in the tomato genome it is considered a stress-induced variant of H1 histone. his1-s mRNA accumulated in vegetative plants in response to other abiotic stress treatments, including application of polyethylene glycol, and salt. The mRNA preferentially accumulated in leaves as compared to roots. his1-s mRNA accumulation was controlled during development; the level was higher in developing seeds of mature green fruit than in detached wilted leaves. H1 histones have been implicated in the general repression of gene expression and in the regulation of specific genes. The rapid accumulation of his1-s mRNA during stress may indicate that this unique, stress-induced H1 histone is involved in controlling gene expression during plant stress.
Similar content being viewed by others
References
Ascenzi R and Gantt JS (1997) A drought-stress-inducible histone gene in Arabidopsis thaliana is a member of a distinct class of plant linker histone variants. Plant Mol Biol 34: 629–641
Ausubel FM (1987) Current protocols in Molecular Biology. New York: John Wiley and Sons
Baker J, Steele C and Dure L III (1988) Sequence and characterization of 6 Lea proteins and their genes from cotton. Plant Mol Biol 11: 277–291
Bouvet P, Dimitrov S, and Wolffe AP (1994) Specific regulation of Xenopus chromosomal 5S rRNA gene transcription in vivo by histone H1. Genes and Development 8: 1147–1159
Bray EA (1988) Drought-and ABA-induced changes in polypeptide and mRNA accumulation in tomato leaves. Plant Physiol 88: 1210–1214
Bray EA (1990) Drought-stress-induced polypeptide accumulation in tomato leaves. Plant Cell Environ 13: 531–538
Bray EA (1993) Molecular responses to water deficit. Plant Physiol. 103: 1035–1040
Bray EA (1997) Plant responses to water deficit. Trends Plant Sci. 2: 48–54
Bray EA, Moses MS, Imai R, Cohen A and Plant ÁL (1993) Gene expression by endogenous abscisic acid during drought stress. In: Close TJ and Bray EA (eds) Plant Responses to Cellular Dehydration During Environmental Stress. Current topics in plant physiology, Vol. 10. Rockville, MD: American Society of Plant Physiologists, pp 167–176
Bray EA, Plant ÁL and Cohen A (1990) Drought-and ABA-regulated gene expression in tomato leaves. In: Bennett AB, O'Neill SD (eds) Horticultural Biotechnology, Plant Biology, Vol. 11. New York: Wiley-Liss, pp 315–322
Bresnick EH, Bustin M, Marsaud V, Richard-Foy H and Hager G (1992) The transcriptionally active MMTV promoter is depleted of histone H1. Nucleic Acids Res 20: 273–278
Chabouté ME, Chaubet N, Gigot C, Phillips G (1993) Histones and histone genes in higher plants: structure and genomic organization. Biochemie 75: 523–531
Chipev CC and Wolffe AP (1992) Chromosomal organization of Xenopus laevis oocyte and somatic 5S rRNA genes in vivo. Mol. Cell. Biol. 12: 45–55
Cohen A and Bray EA (1990) Characterization of three mRNAs that accumulate in wilted tomato leaves in response to elevated levels of endogenous abscisic acid. Planta 182: 27–33
Cohen A, Plant ÁL, Moses MS and Bray EA (1991) Organspecific and environmentally-regulated expression of two abscisic acid-induced genes of tomato. Plant Physiol 97: 1367–1374
Cole RD (1984) A minireview of microheterogeneity in H1 histone and its possible significance. Anal Biochem 136: 24–30
Coles LS and Wells JRE (1985) An H1 histone gene-specific 5’ element and evolution of H1 and H5 genes. Nucleic Acids Res 13: 585–594
Dasso M, Dimitrov S and Wolffe AP (1994) Nuclear assembly is independent of linker histones. Proc Nat Acad Sci USA 91: 12477–12481
Derman E, Krauter K, Walling L, Weinberger C, Ray M and Darnell JE Jr (1981) Transcriptional control in the production of liver-specific mRNAs. Cell 23: 731–739
Devereux J, Hueberli P and Smithies O (1984) A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res 12: 387–395
Felsenstein J (1993) PHYLIP (phylogeny inference package) version 3.5. Department of Genetics, University of Washington, Seattle, WA
Gantt JS and Key JL (1987) Molecular cloning of a pea H1 histone cDNA. Eur J Biochem 166: 119–125
Gantt JS and Lenvik TR (1991) Arabidopsis thaliana H1 histones: Analysis of two members of a small gene family. Eur J Biochem 202: 1029–1039
Goldsbrough AP, Albrecht H and Stratford R (1993) Salicylic acid-inducible binding of a tobacco nuclear protein to a 10-bp sequence which is highly conserved amongst stress-inducible genes. Plant J 3: 563–571
Graziano V, Gerchmann SE, Schneider DK and Ramakrishnan V (1994) Histone H1 is located in the interior of the chromatin 30 nm filament. Nature 368: 351–354
Groudine M, Peretz M and Weintraub H (1981) Transcriptional regulation of hemoglobin switching in chicken embryos. Mol Cell Biol 1: 281–288
Jayawardene N and Riggs CD (1994) Molecular cloning, sequence analysis and differential expression of an introncontaining gene encoding tomato histone H1. Eur J Biochem 223: 693–699
Johnson CA, Goddard JP and Adams RL (1995) The effect of histone H1 and DNA methylation on transcription. Biochem J 305: 791–798
Kahn TL, Fender SE, Bray EA and O'Connell MA (1993) Characterization of expression of drought-and abscisic acid-regulated tomato genes in the drought-resistant species Lycopersicon pennellii. Plant Physiol 103: 597–605
Lång V and Palva ET (1992) The expression of a rab-related gene, rab18, is induced by abscisic acid during the cold acclimation process of Arabidopsis thaliana (L.) Heynh. Plant Mol Biol 20: 951–962
Langridge J, Langridge P and Berquesist PL (1980) Extraction of nucleic acids from agarose gels. Anal Biochem 103: 264–271
Laybourn PJ and Kadonaga JT (1991) Role of nucleosomal cores and histone H1 in regulation of transcription by RNA polymerase II. Science 254: 238–245
Liu J-H and Hill RD 1995. Post-transcriptional regulation of bifunctional α-amylase/subtilisin inhibitor expression in barley embryos by abscisic acid. Plant Mol Bio 29: 1087–1091
Luthe DS and Quatrano RS (1980) Transcription in isolated wheat nuclei. I. Isolation of nuclei and elimination of endogenous ribonuclease activity. Plant Physiol 65: 305–308
Mitcham E, Gross KC and Ng TJ (1989) Tomato fruit cell wall synthesis during development and senescence Plant Physiol 89: 477–481
Mundy J, Yamaguchi-Shinozaki K and Chua N-H (1990) Nuclear proteins bind conserved elements in the abscisic acidresponsive promoter of a rice rab gene. Proc Nat Acad Sci USA 87: 1406–1410
Nakayama T and Iwabuchi M (1993) Regulation of wheat histone gene expression. Crit Rev Plant Sci 12: 97–110
Neill SJ and Horgan R (1985) Abscisic acid production and water relations in wilty tomato mutants subjected to water deficit. J Exp B0t 36: 1222–1231
Ohsumi K, Katagiri C and Kishimoto T (1993) Chromosome condensation in Xenopus mitotic extracts without histone H1. Science 262: 2033–2035
Paranjape SM, Kamakaka RT and Kadonaga JT (1994) Role of chromatin structure in the regulation of transcription by RNA polymerase II. Annu Rev Biochem 63: 265–297
Parry AD, Neill SJ and Horgan R (1988) Xanthoxin levels and metabolism in the wild-type and wilty mutants of tomato. Planta 173: 397–404
Pla M, Goday A, Vilardell J, Gómez J and Pagès M (1989) Differential regulation of the ABA-induced 23–25 kD proteins in embryos and vegetative tissues of viviparous mutants of maize. Plant Mol Biol 94: 1682–1688
Pla M, Gómez J, Goday A and Pagès M (1991) Regulation of the abscisic acid-responsive gene rab28 in maize viviparous mutants. Mol Gen Genet 230: 394–402
Plant ÁL, Cohen A, Moses MS and Bray EA (1991) Nucleotide sequence and spatial expression pattern of a droughtand abscisic acid-induced gene of tomato. Plant Physiol 97: 900–906
Prescott A and Martin C (1987) A rapid method for the quantitative assessment of levels of specific mRNAs in plants. Plant Mol Biol Rep 4: 219–224
Ramakrishnan V, Finch JT, Graziano V, Lee PL, and Sweet RM (1993) Crystal structure of globular domain of histone H5 and its implications for nucleosome binding. Nature 362: 19–223
Razafimahatratra P, Chaubet N, Phillipps G and Gigot C (1991) Nucleotide sequence and expression of a maize H1 histone cDNA. Nucleic Acids Res 19: 1491–1496
Sambrook J, Fritsch EF and Maniatis T (1989) Molecular cloning: A laboratory manual, 2nd Ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
Sanger F, Nicklen S and Coulson AR (1977) DNA sequencing with chain-terminating inhibitors. Proc Nat Acad Sci USA 74: 5463–5467
Schultz TF, Spiker S and Quatrano RS (1996) Histone H1 enhances the DNA binding activity of the transcription factor EmBP-1. J Biol Chem 271: 25742–25745
Schwartz RM and Dayhoff MO (1978) Matrices for detecting distant relationships. In: Dayhoff MO (ed) Atlas of Protein Sequence and Structure. Washington, DC: National Biomedical Research Foundation, pp 353–358
Shen X and Gorovsky MA (1996) Linker histone H1 regulates specific gene expression but not global transcription in vivo. Cell 86: 475–483
Shen X, Yu L, Weir JW and Gorovsky MA (1995) Linker histones are not essential and affect chromatin condensation in vivo. Cell 82: 47–56
Shinozaki K and Yamaguchi-Shinozaki K (1997) Gene expression and signal transduction in water-stress response. Plant Physiol 115: 327–334
Spiker S (1988) Histone variants and high mobility group nonhistone chromosomal proteins of higher plants: their potential for forming a chromatin structure that is either poised for transcription or transcriptionally inert. Physiol Plant 75: 200–213
Szekeres M, Haizel T, Adam E and Nagy F (1995) Molecular characterization and expression of a tobacco histone H1 cDNA. Plant Mol Biol 27: 597–605
Thompson AJ and Corlett JE (1995) mRNA levels of four tomato (Lycopersicon esculentum Mill. L.) genes related to fluctuating plant and soil water status. Plant Cell Environ 18: 773–780
Travers AA (1994) Chromatin structure and dynamics. BioEssays 16: 657–662
Van Holde KE (1988) Chromatin. New York: Springer-Verlag
Walling L, Drews GN and Goldberg RB (1986) Transcriptional and post-transcriptional regulation of soybean seed protein mRNA levels. Proc Natl Acad Sci USA 83: 2123–2127
Wei T and O'Connell MA (1996) Structure and characterization of a putative drought-inducible H1 histone gene. Plant Mol Biol 30: 255–268
Wells D and Brown D (1991) Histone and histone gene compilation and alignment update. Nucleic Acids Res 19S: 2173–2188
Williamson JD and Quatrano RS (1988) ABA-regulation of two classes of embryo-specific sequences in mature wheat embryos. Plant Physiol 86: 208–215
Wolffe AP (1994) Transcription: In tune with the histones. Cell 77: 13–16
Yang P, Katsura M, Nakayama T, Mikami K and Iwabuchi M (1991) Molecular cloning and nucleotide sequences of cDNAs for histone H1 and histone H2B variants from wheat. Nucleic Acids Res 19: 5077
Zeevaart JAD and Creelman RA (1988) Metabolism and physiology of abscisic acid. Annu Rev Plant Physiol PlantMol Biol 39: 439–473
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bray, E.A., Shih, TY., Moses, M.S. et al. Water-deficit induction of a tomato H1 histone requires abscisic acid. Plant Growth Regulation 29, 35–46 (1999). https://doi.org/10.1023/A:1006264001528
Issue Date:
DOI: https://doi.org/10.1023/A:1006264001528