Abstract
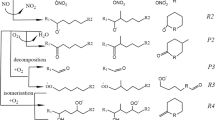
Measurements show that 20–60% of the carbon mass present in fine atmospheric particulate matter consists of water soluble organic compounds (WSOC). However, only 5–20% of this WSOC has been identified, mainly as dicarboxylic acids. Because of their high solubility in water, multifunctional secondary compounds derived from the gas-phase oxidation of volatile organic compounds (VOC) are suspected to be key contributors to the WSOC. To test this assumption, an estimate of aqueous uptake of secondary VOC was included in a highly detailed gas-phase mechanism which treats explicitly the formation of the secondary VOC from a set of representative primary species. Simulations were conducted for 2 scenarios, representing typical rural and urban areas. It was observed that the uptake of secondary VOC can lead to WSOC mass concentrations in the range of a few μC m−3, in fairly good agreement with typical WSOC mass concentrations measured. Speciation of WSOC was found to be mainly as tri- or higher multifunctional hydroxy-carbonyl species and hydroxy-hydroperoxide-carbonyl species, in urban and rural environments, respectively. However, it was also found that taking into account only the absorption of secondary VOC does not bring the carboxylic acids mass concentration in agreement with measurements. An attempt was made to explain this discrepancy by introducing chemistry occurring within deliquescent aerosols.
Similar content being viewed by others
References
Atkinson, R., 1994: Gas-phase tropospheric chemistry of organic compounds, J. Phys. Chem. Ref. Data, Monograph 2, 1-216.
Aumont, B., Madronich, S., Ammann, M., Kalberer, M., Baltensperger, U., Hauglustaine, D., and Brocheton, F., 1999: On the NO2 + soot reaction in the atmosphere, J. Geophys. Res. 104, 1729-1736.
Bey, I., 1997: Contribution des processus nocturnes a la chimie tropospherique: Modelisation des flux de radicaux et transformation des precurseurs d'ozone (COV, NOx), PhD thesis, University Paris 12.
Bey, I., Aumont, B., and Toupance, G., 1997: The nighttime production of OH radicals in the continental troposphere, Geophys. Res. Lett. 24, 1067-1070.
Buxton, G. V., Greenstock, C. L., Helman, W. P., and Ross, A. B., 1988: Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (OH/O?) in aqueous solution, J. Phys. Chem. Ref. Data 17, 513-883.
Cadle, S. H. and Groblicki, P. J., 1982: An evaluation of methods for the determination of organic and elemental carbon in particulate samples, in G. T. Wolff and R. L. Klimisch (eds), Particulate Carbon-Atmospheric Life Cycle, Plenum Press, New York, pp. 89-109.
Carter, W. P. L., 1990: A detailed mechanism for the gas-phase atmospheric reactions of organic compounds, Atmos. Environ. 24, 481-518.
Chameides, W. L. and Davis, D. D., 1983: Aqueous-phase source of formic acid in clouds, Nature 304, 427-429.
Faust, B. C. and Zepp, R. G., 1993: Photochemistry of aqueous Iron(III)-polycarboxylate complexes: Role in the chemistry of atmospheric and surface waters, Environ. Sci. Technol. 27, 2517-2522.
Faust, B. C., 1994: Photochemistry of clouds, fogs and aerosols, Environ. Sci. Technol. 28, 217A-222A.
Gery, M. W., Whitten, G. Z., and Killus, J. P., 1988: Development and testing of the CBM-IV for urban and regional modeling, EPA Report, Contract Nr. 68-02-41136.
Grosjean, D., 1992: In situ organic formation during smog episodes: Estimated production and chemical functionality, Atmos. Environ. 26A, 953-963.
Grosjean, D., Van Cauwenberghe, K., Schmid, J. P., Kelley, P. E., and Pitts, J. N., 1978: Identification of C3-C10 aliphatic dicarboxylic acids in airborne particulate matter, Environ. Sci. Technol. 12, 313-317.
Hough, A. M., 1986: The production of photochemical pollution in southern England and the effect of vehicle exhaust emission control strategies, AERE Report, Nr. R-12069, Harwell Laboratory.
Jacob, D., 1986: Chemistry of OH in remote clouds and its role in the production of formic acid and peroxymonosulfate, J. Geophys. Res. 91, 9807-9826.
Jacob, D., Gottlieb, E. W., and Prather, M. J., 1989: Chemistry of polluted cloudy boundary layer, J. Geophys. Res. 94, 12975-13002.
Kames, J. and Schurath, U., 1992: Alkyl nitrates and bifunctional nitrates of atmospheric interest: Henry's law constants and their temperature dependencies, J. Atmos. Chem. 15, 79-95.
Kames, J. and Schurath, U., 1995: Henry's law and hydrolysis of peroxyacetyl nitrates (PANs) using a homogeneous gas-phase source, J. Atmos. Chem. 21, 151-164.
Kawamura, K. and Kaplan, I. R., 1987: Motor exhaust emissions as a primary source of dicarboxylic acids in Los Angeles ambiant air, Environ. Sci. Technol. 21, 105-110.
Kim, Y. P., Seinfeld, J. H., and Saxena, P., 1993: Atmospheric gas-aerosol equilibrium I. Thermodynamic model, Aerosol Sci. Technol. 19, 157-181.
Madronich, S. and Calvert, J. G., 1989: The NCAR master mechanism of the gas-phase chemistry-Version 2.0, Rep. NCAR/TN-333+STR, National Center for atmospheric Research.
Madronich, S. and Calvert, J. G., 1990: Permutation reactions of organic peroxy radicals in the troposphere, J. Geophys. Res. 95, 5697-5715.
Middleton, P., Stockwell, W. R., and Carter, W. P. L., 1990: Aggregation and analysis of volatile organic compounds emissions for regional modeling, Atmos. Environ. 24, 1107-1133.
Mueller, P. K., Hidy, G. M., Baskett, R. L., Fung, K. K., Henry, R. C., Lavery, T. F., Waren, K. K., and Watson, J. G., 1983: The sulfate regional experiment: Report of findings, Report EA-1901, Electric Power Reasearch Institute, Palo Alto, CA 94306, Vol. 2.
Mueller, P. K., Fung, K. K., Heisler, S. L., Grojean, D., and Hidy, G. M., 1982: Atmospheric particulate carbon observations in urban and rural areas of the United States, in G. T. Wolff and R. L. Klimisch (eds), Particulate Carbon-Atmospheric Life Cycle, Plenum Press, New York, pp. 343-370.
Nathanson, G. M., Davidovits, P., Worsnop, D. R., and Kolb, C. E., 1996: Dynamics and kinetics at the gas-liquid interface, J. Phys. Chem. 100, 13007-13020.
Odum, J. R., Jungkamp, T. P. W., Griffin, R. J., Flagan, R. C., and Seinfeld, J. H., 1997: The atmospheric aerosol-forming potential of whole gasoline vapor, Science 276, 96-99.
O'Sullivan, D. W., Lee, M., Noone, B. C., and Heikes, B. G., 1996: Henry's law constant determinations for hydrogen peroxide, methyl hydroperoxide, hydroxymethyl hydroperoxide, ethyl hydroperoxide, and peroxyacetic acid, J. Phys. Chem. 100, 3241-4247.
Rogge, W. F., Mazurek, M. A., Hildemann, L. M., Cass, G. R., and Simoneit, B. R. T., 1993: Quantification of urban organic aerosols at a molecular level: Identification, abundance and seasonal variation, Atmos. Environ. 27A, 1309-1330.
Sander, R., 1999: Compilation of Henry's Law Constants for inorganic and organic species of potential importance in environmental chemistry (Version 3), http://www.mpchmainz. mpg.de/_sander/res/henry.html.
Satsumabayashi, H., Kurita, H., Yokouchi, Y., and Ueda, H., 1990: Photochemical formation of particulate dicarboxylic acids under long range transport in central Japan, Atmos. Environ. 24A, 1443-1450.
Saxena, P., Hildemann, L. M., McMurry, P. H., and Seinfeld, J. H., 1995: Organics alter hygroscopic behavior of atmospheric particles, J. Geophys. Res. 100, 18755-18770.
Saxena, P. and Hildemann, L. M., 1996: Water-soluble organics in atmospheric particles: A critical review of the literature and application of thermodynamics to identify candidate compounds, J. Atmos. Chem. 24, 57-109.
Schwartz, S., 1986: Mass transport considerations pertinent to aqueous phase reactions of gases in liquid waters clouds, in W. Jaeschke (ed.), Chemistry of Multiphase Systems, Springer, pp. 415-471.
Seinfeld, J. H. and Pandis, S. N., 1998: Atmospheric Chemistry and Physics, Wiley, New York.
Sempere, R. and Kawamura, K., 1994: Comparative distributions of dicarboxylic acids and related polar compounds in snow, rain, and aerosols from urban atmosphere, Atmos. Environ. 28, 449-459.
Shepson, P. B., Mackay, E., and Muthuramu, K., 1996: Henry's law constants and removal processes for several atmospheric β-hydroxy alkyl nitrates, Environ. Sci. Technol. 30, 3618-3623.
Sillman, S., Logan, J. A., and Wofsy, S. C., 1990: The sensitivity of ozone to nitrogen oxides and hydrocarbons in regional ozone episodes, J. Geophys. Res. 95, 1837-1851.
Simpson, D., 1993: Photochemical model calculations over Europe for two extended summer episodes: 1985 and 1989. Model results and comparison with observations, Atmos. Environ. 27A, 921-943.
Simpson, D., Guenther, A., Hewitt, C. N., Steinbrecher, R., 1995: Biogenic emissions in Europe: 1. Estimates and uncertainties, J. Geophys. Res. 100, 22875-22890.
Stockwell, W. R., Middleton, P., Chang, J. S., Tang, X., 1990: The second generation Regional Acid Deposition Model chemical mechanism for regional air quality modeling, J. Geophys. Res. 95, 16343-16367.
Suzuki, T., Ohtaguchi, K., and Koide, K., 1992: Application of principal components analysis to calculate Henry's constant from molecular structure, Comp. Chem. 16, 41-52.
Wesely, M. L., 1989: Parametrization of surface resistance to gaseous dry deposition in regional scale numerical models, Atmos. Environ. 23, 1293-1304.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Aumont, B., Madronich, S., Bey, I. et al. Contribution of Secondary VOC to the Composition of Aqueous Atmospheric Particles: A Modeling Approach. Journal of Atmospheric Chemistry 35, 59–75 (2000). https://doi.org/10.1023/A:1006243509840
Issue Date:
DOI: https://doi.org/10.1023/A:1006243509840