Abstract
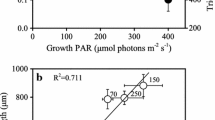
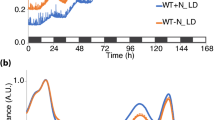
The daily cycle of nitrogenase expression in the marine filamentous nonheterocystous cyanobacterium Trichodesmium spp. is controlled by a circadian rhythm. We evaluated the rhythm of two key photosynthesis genes, psbA of photosystem II and psaA of photosystem I, in Trichodesmium sp. IMS 101 using the 3 criteria for an endogenous rhythm. The transcript abundance of psbA and psaA transcripts oscillated with a period of ca. 24 h under a 12 h light/12 h dark regime. At 24 °C and 28 °C the cyclic pattern of transcript abundance was maintained for at least 58 h under constant light conditions, whereas the periods were about 24 h at 24 °C, and 26–30 h at the higher temperature. The cycles of psbA and psaA gene expression were entrained using light-dark cues. Transcription of nifHDK was initiated prior to the light period, followed by psbA and finally psaA. There was a 90° (6 h) phase difference between the net accumulation of nifHDK and psbA transcripts, as well as between that of psbA and psaA transcripts. Results of inhibitor experiments indicated that psbA and psaA transcription was regulated differently by initiation and degradation during the light period. Short-term changes of light conditions resulted in significant effects on psbA transcription and nitrogenase activity, but had less of an effect on psaA and nifHDK transcription.
Similar content being viewed by others
References
Anderson, B. and Styring, S. 1991. Photosystem II: molecular organization, function and acclimation. Curr. Top. Bioenerg. 16: 1-81.
Ausubel, F.M., Brent, R., Kingston, R.E., Moore, D.D., Seidman, J.G., Smith, J.A. and Struhl, K. (Eds.), 1990. Current Protocols in Molecular Biology, Greene Publishing/Wiley-Interscience, New York.
Bergman, B.B., Gallon, J.R., Rai, A.N. and Stal, L.J. 1997. N2 fixation by non-heterocystous cyanobacteria. FEMS Microbiol. Rev. 19: 139-185.
Bustos, S.A., Schaefer, M.R. and Golden, S.S. 1990. Different and rapid responses of four cyanobacterial psbA transcripts to changes in light intensity. J. Bact. 172: 1998-2004.
Cantrell, A. and Bryant, D.A. 1987. Molecular cloning and nucleotide sequence of the psaA and psaB genes of the cyanobacterium Synechococcus sp. PCC 7002. Plant Mol. Biol. 9: 453-468.
Capone, D.G. 1993. Determination of nitrogenase activity in aquatic samples using the acetylene reduction procedure. In: P.F. Kemp, B.F. Sherr, E.B. Sherr and J.J. Cole (Eds.), Current Methods in Aquatic Microbiology, Lewis Publishers, New York, pp. 621-631.
Capone, D.G., O'Neil, J.M., Zehr, J. and Carpenter, E.J. 1990. Basis for diel variation in nitrogenase activity in the marine planktonic cyanobacterium Trichodesmium thiebautii. Apll. Environ. Microbiol. 56: 3532-3536.
Capone, D.G., Zehr, J.P., Paerl, H.W., Bergman, B. and Carpenter, E. 1997. Trichodesmium, a globally significant marine cyanobacterium. Science 276: 1221-1229.
Carpenter, E.J. 1983. Physiology and ecology ofmarine Oscillatoria (Trichodesmium). Mar. Biol. Lett. 4: 69-85.
Carpenter, E.J. and Price, C.C. 1976. Marine Oscillatoria (Trichodesmium): explanation for aerobic nitrogen fixation without heterocysts. Science 19: 1278-1280.
Chen, Y.-B., Zehr, J.P. and Mellon, M.T. 1996. Growth and nitrogen fixation of the diazotrophic filamentous nonheterocystous cyanobacterium Trichodesmium sp. IMS 101 in defined media: evidence for a circadian rhythm. J. Phycol. 32: 916-923.
Chen, Y.-B., Dominic, B., Mellon, M.T. and Zehr, J.P. 1998. Circadian rhythm of nitrogenase gene expression in the filamentous nonheterocystous cyanobacterium Trichodesmium sp. IMS 101. J. Bact. 180: 3598-3605.
Colón-López, M.S. and Sherman, L.A. 1998. Transcriptional and translational regulation of photosystem I and II genes in lightdark-and continuous-light-grown cultures of the unicellular cyanobacterium Cyanothece sp. strain ATCC 51142. J. Bacteriol. 180: 519-526.
Colón-López, M.S., Sherman, D.M. and Sherman, L.A. 1997. Transcriptional and translational regulation of nitrogenase in light-dark-and continuous-light-grown cultures of the unicellular cyanobacterium Cyanothece sp. strain ATCC 51142. J. Bacteriol. 179: 4319-5327.
Constant, S., Perewoska, I., Alfonso, M. and Kirilovsky, D. 1997. Expression of the psbA gene during photoinhibition and recovery in Synechocystis PCC 6714: inhibition and damage of transcriptional and translational machinery prevent the restoration of photosystem II activity. Plant Mol. Biol. 34: 1-13.
Doran, E. and Cattolico, R.A. 1997. Photoregulation of chloroplast gene transcription in the comophytic alga Heterosigma carterae. Plant Physiol. 115: 773-781.
Frederiksson, C. 1996. Nitrogenase localisation reveals cell differentiation in filamentous, non-heterocystous cyanobacteria. Stockholm University.
Gallon, J.R., Jones, D.A. and Page, T.S. 1996. Trichodesmium, the paradoxical diazotroph. Algol. Stud. 83: 215-243.
Golbeck, J.H. 1992. Structure and function of photosystem I. Annu. Rev. Plant Physiol. Plant Mol. Biol. 43: 293-324.
Golden, S.S. 1994. Light-responsive gene expression and the biochemistry of the photosystem II reaction center. In: D.A. Bryant (Ed.), The Molecular Biology of Cyanobacteria, Kluwer Academic Publishers, Dordrecht, Netherlands, pp. 693-714.
Golden, S.S. 1995. Light-responsive gene expression in cyanobacteria. J. Bacteriol. 177: 1651-1654.
Golden, S.S., Ishiura, M., Johnson, C.H. and Kondo, T. 1997. Cyanobacterial circadian rhythms. Annu. Rev. Plant Physiol. Plant Mol. Biol. 48: 327-354.
Ho, K.K. and Krogman, D.W. 1982. Photosynthesis. In: N.G. Carr and B.A. Whitton (Eds.), The biology of Cyanobacteria, Blackwell Scientific Publications, Oxford, pp. 191-214.
Huang, S., Kawazoe, R. and Herrin, D.L. 1996. Transcription of tufA and other chloroplast-encoded genes is controlled by a circadian clock in Chlamydomonas. Proc. Natl. Acad. Sci. USA 93: 993-1000.
Johnson, C.H. and Hastings, J.W. 1986. The elusive mechanism of the circadian clock. Am. Scient. 74: 29-36.
Kondo, T., Strayer, C.A., Kulkarni, R.D., Taylor, W.I.M., Golden, S.S. and Johnson, H. 1993. Circadian rhythms in prokaryotes: luciferase as a reporter of circadian gene expression in cyanobacteria. Proc. Natl. Acad. Sci. USA 90: 5672-5676.
Kulkarni, R.D., Schaefer, M.R. and Golden, S.S. 1992. Transcriptional and posttranscriptional components of psbA response to high light intensity in Synechococcus sp. strain PCC 7942. J. Bact. 174: 3775-3781.
Lönneborg, A., Kalla, S., Samuelsson, G., Gustafsson, P. and Öquist, G. 1988. Light-regulated expression of the psbA transcript in the cyanobacterium Anacystis nidulans. FEBS Lett. 240: 110-114.
Mitsui, A., Kumazawa, S., Takahashi, A., Ikemoto, H., Cao, S. and Arai, T. 1986. Strategy by which nitrogen-fixing unicellular cyanobacteria grow photoautotrophically. Nature 323: 720-722.
Mohamed, A. and Jansson, C. 1989. Influence of light on accumulation of photosynthesis-specific transcripts in the cyanobacterium Synechocystis 6803. Plant Mol. Biol. 13: 693-70.
Mohamed, A. and Jansson, C. 1991. Photosynthesis electron transport controls degradation but not reduction of psbA transcripts in the cyanobacterium Synechocystis 6803. Plant Mol. Biol. 16: 891-897.
Morden, C.W. and Golden, S.S. 1989. psbA genes indicate common ancestry of prochlorophytes and chloroplasts. Nature 337: 382-385.
Nyhus, K.J., Ikeuchi, M., Inoue, Y., Whitmarsh, J. and Pakrasi, H.B. 1992. Purification and characterization of photosystem I complex from the filamentous cyanobacterium Anabaena variabilis ATCC 29413. J. Biol. Chem. 267: 12489-12495.
Ohki, K. and Fujita, Y. 1992. Light-dependent maintenance of active nitrogenase in the non-heterocystous cyanophyte Trichodesmium sp. NIBB 1067. In: N. Murata (Ed.), Research in Photosynthesis, Kluwer Academic Publishers, Dordrecht, Netherlands, pp. 103-106.
Paerl, H.W. 1994. Spatial segregation of CO2 fixation in Trichodesmium spp.: linkage to N2 fixation potential. J. Phycol. 30: 790-799.
Prufert-Bebout, L., Paerl, H.W. and Lassen, C. 1993. Growth, nitrogen fixation, and spectral attenuation in cultivated Trichodesmium species. Appl. Environ. Microbiol. 59: 1367-1375.
Sambrook, J., Fritsch, E.F. and Maniatis, T. (Eds.), 1989.Molecular Cloning: A Laboratory Manual, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
Schweiger, H.G., Hartwig, R. and Schweiger, M. 1986. Cellular aspects of circadian rhythms. J. Cell. Sci. Suppl. 4: 181-200.
Smart, L.B. and Mclntosh, L. 1991. Expression of photosynthesis genes in the cyanobacterium Synechocystis sp. PCC 6803: psaApsaB and psbA transcripts accumulate in dark-grown cells. Plant Mol. Biol. 17: 959-971.
Stal, L.J. and Krumbein, W.E. 1987. Temporal separation of nitrogen fixation and photosynthesis in the filamentous, nonheterocystous cyanobacterium Oscillatoria sp. Arch. Microbiol. 149: 76-80.
Tandeau De Marsac, N. and Houmard, J. 1988. Complementary chromatic adaptation: physiological conditions and action spectra. In: L. Packer and A.N. Glazer (Eds.), Cyanobacteria, Academic Press, San Diego/New York/Tokyo, pp. 318-328.
Tyystjärvi, T., Mulo, P., Mäenpää, P. and Aro, E.-M. 1996. D1 polypeptide degradation may regulate psbA gene expression at transcriptional and translational levels in Synechocystis sp. PCC 6803. Photosynth. Res. 47: 111-120.
Vermaas, W.J.F. and Ikeuchi, M. 1991. Photosystem II. In: L. Bogorad and I.K. Vasil (Eds.), The Photosynthetic Apparatus: Molecular Biology and Operation, Academic Press, San Diego, CA, pp. 25-111.
Walsby, A.E. (1992. The gas vesicles and buoyancy of Trichodesmium. In: E.J. Carpenter, D. Capone and J.G. Rueter (Eds.),Marine Pelagic Cyanobacteria: Trichodesmium and Other Diazotrophs, Kluwer, Dordrecht, Netherlands, pp. 141-161.
Wilkins, N.B. 1992. Circadian rhythms: their origin and control. New Phytol. 121: 347-375.
Zehr, J.P. and McReynolds, L.A. 1989. Use of degenerate oligonucleotides for amplification of the nifH gene from the marine cyanobacterium Trichodesmium spp. Appl. Environ. Microbiol. 55: 2522-2526.
Zehr, J.P., Wyman, M., Miller, V., Duguay, L. and Capone, D.G. 1993. Modification of the Fe protein of nitrogenase in natural populations of Trichodesmium thiebautii. Appl. Environ. Microbiol. 59: 669-676.
Zehr, J.P., Dominic, B., Chen, Y.-B., Mellon, M.T. and Meeks, J.C. 1998. Nitrogen fixation in the marine cyanobacteria Trichodesmium: a challenging model for ecology and molecular biology. In: G.A. Peschek, W. Loffelhardt and G. Schmetterer (Eds.), Phototrophic Prokaryotes, Plenum Press, New York, pp. 485-500.
Zurawski, G., Bohnert, H.J., Whitefeld, P.R. and Bottomley, W. 1982. Nucleotide sequence of the gene for the Mr 32,000 thylakoid membrane protein from Spinacea oleracea and Nicotiana debneyi predicts a totally conserved primary translation product of Mr 38,950. Proc. Natl. Acad. Sci. USA 79: 7699-7703.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Chen, YB., Dominic, B., Zani, S. et al. Expression of photosynthesis genes in relation to nitrogen fixation in the diazotrophic filamentous nonheterocystous cyanobacterium Trichodesmium sp. IMS 101. Plant Mol Biol 41, 89–104 (1999). https://doi.org/10.1023/A:1006231805030
Issue Date:
DOI: https://doi.org/10.1023/A:1006231805030